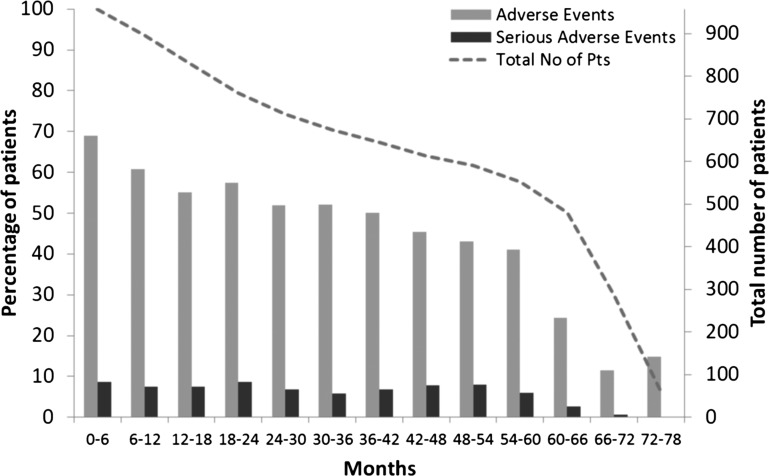
Figure 2.
Percentage of patients experiencing adverse events (AEs) and serious AEs over each 6-month period from the start of the feeder study (safety population; N=958). Note, sharp decline in patient numbers from month 60 is due to per protocol site closure in countries where certolizumab pegol became commercially available.