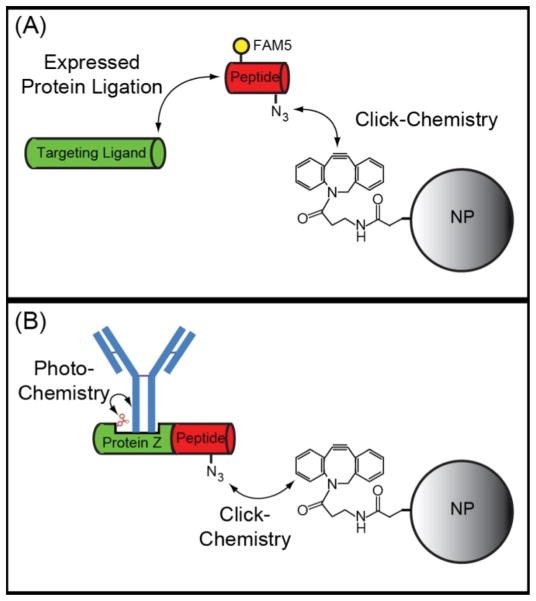
Figure 2.
Schematic of EPL-click chemistry bioconjugation techniques. (A) EPL results in the chemoselective attachment of peptides functionalized with an azide group to recombinantly expressed targeting ligands. Additional functional groups such as fluorescent dyes (e.g. FAM5) can also be included on the peptide. The azido-labeled targeting ligands can then be efficiently and site-specifically attached to alkyne-modified nanoparticles (NP) via click chemistry. The constrained alkyne, Aza-dibenzocyclooctyne, is shown, which allows for copper-free click chemistry. (B) The EPL-click chemistry approach can be combined with non-natural amino acid incorporation to produce an antibody binding protein, Protein Z, with a photocrosslinker (e.g. benzoylphenylalanine) in the Fc binding domain. This allows for the site-specific and covalent attachment of full-length antibodies to nanoparticles.