Table 2. Substrate scope a , b , c , d .
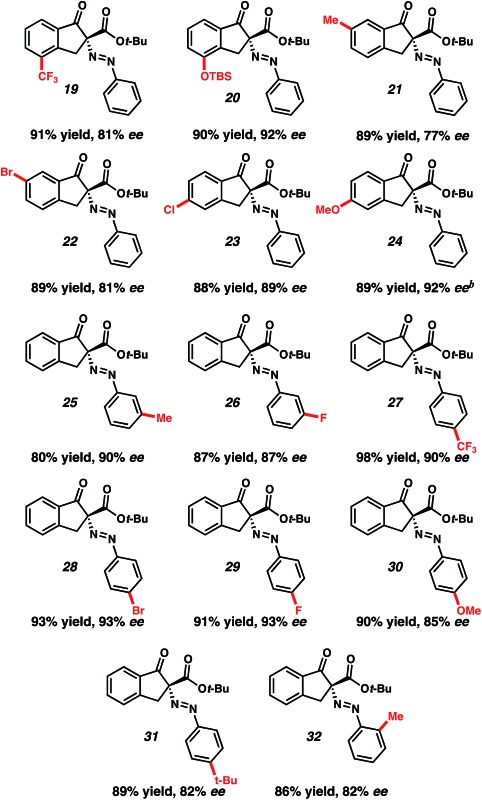
|
aConditions: nucleophile (1 equiv.), 16 (10 mol%), NaH2PO4 (6 equiv.), ArN2BF4 (1.2 equiv.), cyclohexane (0.025 M).
bConditions: nucleophile (1 equiv.), 17 (10 mol%), NaH2PO4 (6 equiv.), ArN2BF4 (1.2 equiv.), MTBE (0.025 M).
cIsolated yield.
dEnantiomeric excess determined by chiral phase HPLC.
