Synopsis
Diabetes is the leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) in the United States. There was an estimated 7 million cases of diabetic kidney disease (DKD) in the last National Health and Nutrition Examination survey (2005-2008). High blood pressure, hyperglycemia and other metabolic abnormalities interactively promote DKD, thus there has been increasing interest and renewed focus on the metabolic dysregulation and the interactions between heart and kidney pathologies observed in DKD. Indeed metabolic abnormalities that are observed in overweight or obese individuals are known to impact blood pressure regulation in kidney and cardiovascular disease; e.g. cardiorenal metabolic syndrome. In this context, obesity has been associated with increased blood pressure variability and nocturnal non-dipping which are risk predictors for albuminuria and DKD. These collective metabolic abnormalities have also been observed in earlier stages of DKD in association with micro-albuminuria. Herein we review the current literature regarding the role of blood pressure variability and nocturnal non-dipping of blood pressure as well as the presence of DKD, in the absence of albuminuria, as risk predictors for progressive DKD. We also discuss the importance of glycemic and blood pressure control in patients with diabetes and CKD, and the use of oral hypoglycemic agents and anti-hypertensive agents in this patient cohort.
Keywords: Albuminuria, Proteinuria, Diabetes, Cardiorenal Syndrome, Diabetic Nephropathy, Chronic Kidney Disease, Blood Pressure Variability
Introduction
Prevalent chronic kidney disease (CKD) in the United States (US) has increased over last few decades and comprises an alarming 13% of the US general population (1). Diabetes is recognized as the leading cause of CKD and end stage renal disease (ESRD), and accounts for about 40% of ESRD cases in the US (2-4). It is estimated that CKD affects more than 35% of adults with diabetes and nearly 20% of adults with hypertension (5). The expansion of CKD can be explained, in part, by the increased prevalence of obesity and diabetes, thus raising concerns for even more pronounced trends in the future (1). Regardless of etiology, CKD is prevalent enough to be considered a critical public health concern, especially with the associated increased cardiovascular disease (CVD) morbidity and mortality (1, 6). In this context, diabetic kidney disease (DKD) is a clinical syndrome characterized by early glomerular hyperfiltration and albuminuria, followed by increasing proteinuria and a decline in glomerular filtration rate (GFR), blood pressure elevation, and high risk of CVD morbidity and mortality (7). The precise etiology of this disease, although increasingly common due to the global expansion of diabetes and obesity, is poorly understood.
Pathology of DKD
DKD has been studied extensively over the years , but our understanding of this complex disease process is far from complete. It is generally accepted that diabetes is associated with diverse structural changes in the kidney; in fact all structural compartments are affected, leading to functional impairments at all levels of the nephron. Three basic steps have been described in progression of DKD (8); 1) Glomerular hypertrophy and hyperfiltration, 2) Inflammation of glomeruli and tubulointerstital area, 3) Apoptosis of cells and accumulation of extracellular matrix.
The hyperglycemia observed in diabetes contributes to a micro inflammatory, oxidative stress milleau and extracellular matrix expansion within the kidney (8). There are three critical abnormalities including intracellular metabolism, formation of advanced glycation end-products, and intra-glomerular hypertension implicated in development of glomerular endothelial and mesangialcell injury. These pathological changes are associated with cellular injury, expression of adhesion molecules and macrophage infiltration in kidney tissue (8).
Expansion of the mesangium, thickening of the glomerular basement membrane, and hyalinosis of afferent and efferent arterioles are the characteristic lesions of DKD (9). It is generally thought that thickening of GBM and expansion of mesangium occur early in course of diabetes. Diffuse global mesangial expansion is seen in diabetes, and it is primarily due to increase in extracellular matrix, with relatively little contribution from increase in mesangial cell volume (9). Kimmelstiel-Wilson nodules (acellular to pauci-cellular nodular accumulations of mesangial matrix) have been described in DKD. These nodular sclerotic lesions occur in patients with advanced DKD, and their presence is considered to mark transition from early to more advanced stages of DKD (10). Kimmelstiel-Wilson nodules are not pathognomonic of DKD as these lesions can be seen in other conditions like monoclonal immunoglobulin deposition disorders, membrano-proliferative glomerulonephritis, post-infectious glomerulonephritis and amyloidosis (9, 10). In parallel, hyaline deposition in the glomerular arterioles is another typical histological feature of DKD. Hyalinosis and resultant hyaline appearance (homogeneous and glassy) is due to insinuation of plasma proteins into vascular wall.
Alternatively, loss of integrity of the filtration barrier and podocyte injury with effacement of foot processes and loss of podocytes are other microscopic changes evident in DKD that play important roles in the development of progressive sclerosis and proteinuria (9).
Recently, DKD in Type1 and Type 2 DM has been classified based on severity of glomerular lesions. Classification based on glomerular lesions has been chosen over interstitial or vascular lesion, due to ease of recognition and good inter-observer reproducibility. Additionally, it has been suggested that severity of chronic interstitial and glomerular lesions co-relate closely. The pathological classification of DKD as proposed by Renal Pathology Society (10): Class 1- Diabetic injury with GBM thickening (> 2 standard deviations from normal), Class 2 Mesangial expansion, 2a Mild mesangial expansion, 2b Severe Mesangial expansion, Class 3-Nodular sclerosis (Kimmelstiel Wilson lesion), Class 4- Advanced diabetic glomerulosclerosis: global sclerosis involving > 50% of glomeruli in addition to the above changes. Ongoing basic science and clinical research is helping shape our understanding of DKD pathogenesis and correlation between histological lesions of DKD and progression of clinical DKD.
The Cardiorenal Syndrome (CRS) and DKD
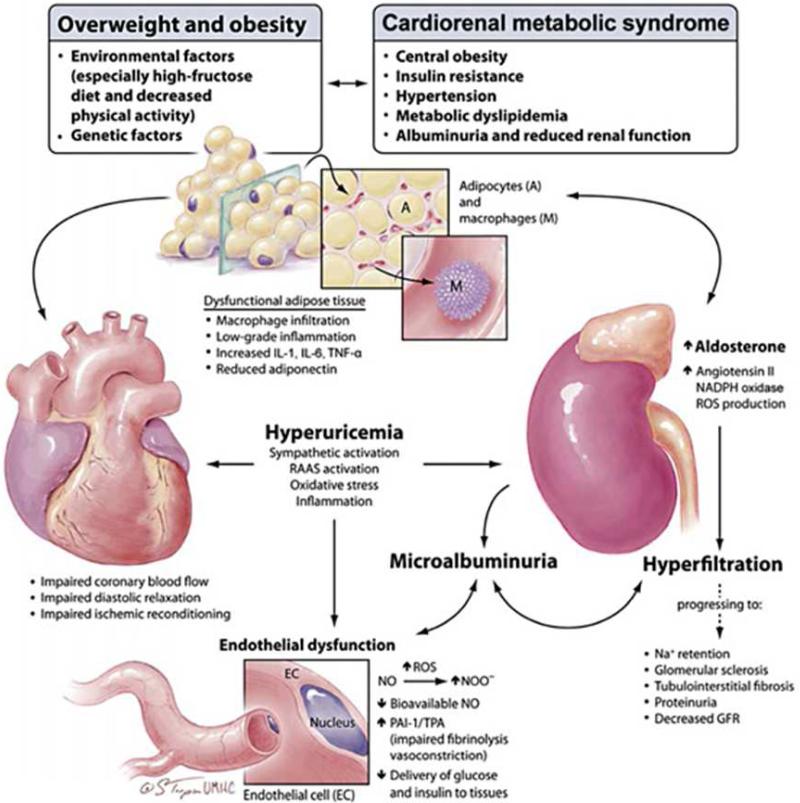
Involvement of both kidneys and the cardiovascular system is common in conjunction with overweight/obesity, metabolic abnormalities, hypertension and early type 2 diabetes (T2DM). Thus, it is important to understand how involvement of one organ system contributes to the dysfunction of the other and these complex interactions have been captured with the emergence of the concept of cardiorenal syndrome (CRS) (7, 11-13). Risk factors that influence heart and kidney disease like overweight or obesity, hypertension, insulin resistance, and metabolic dyslipidemic function are the defining components of CRS (Fig 1) (11). In and of itself, the presence of hypertension, obesity and hyperinsulinemia are independently associated with reductions in kidney function (12). The interaction of these factors and their metabolic and immunological effect should be referred to as the CRS. It is well described that obesity is associated with altered intra-renal physical forces, inappropriate activation of the renin-angiotensin system (RAS) and sympathetic nervous system, and decreased activity of endogenous natriuretic peptides that contribute to elevations in blood pressure and altered responses to handling of glucose in individuals with insulin resistance (14). Thus the various components of CRS interact via complex intertwined pathways and result in the loss of renal structure and function.
Figure 1.
The interrelationship between adiposity and maladaptive changes in the heart and kidney in CRS. GFR = Glomerular filtration rate; IL = interleukin; PAI = plasminogen activator inhibitor; RAAS = renin-angiotensin-aldosterone system; ROS = reactive oxygen species; TNF = tumor necrosis factor; TPA = tissue plasminogen activator. From Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. The Journal of pediatrics. 2009; 155: 049, with permission. Copyright © 2011 Karger Publishers, Basel, Switzerland.
Impact of hypertension on DKD
There have been a number of seminal studies describing the importance of hypertension to cardiovascular mortality in individuals with DKD. In this context, approximately 66% of individuals with an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73m2 have hypertension and as eGFR diminishes over time, the prevalence rates increase from 36% in Stage 1 to 84% in Stages 4-5 CKD (15). As elevations in blood pressure dictate to some extent cardiovascular mortality, it has been noted that mortality due to CVD is 10 to 30 times higher in individuals with kidney disease compared to the general population a relationship that extends into earlier stages of DKD (16). This relationship has been described as a continuous relationship--with reductions in glomerular filtration rate (GFR) and increases in proteinuria come a graded increase in CVD (17). Moreover, recent studies support the notion that even early stages of CKD pose a significant risk of CVD (18).
Blood pressure control in diabetes has been studied extensively and stricter blood pressure targets have been tested overtime. Many studies have demonstrated the beneficial effects of blood pressure control on various outcomes in patients with diabetes; however, blood pressure targets have been a source of debate for several years (19). There is sufficient data to support blood pressure control in T2DM because this control reduces proteinuria and progression of DKD (20). The United Kingdom Prospective Diabetes study (UKPDS) suggests the potential micro-vascular benefits of blood pressure control in subjects with diabetes, wherein 758 subjects with T2DM were randomized to tight blood pressure control (<150/85 mmHg) and 390 to less tight control (<180/105 mmHg). Mean blood pressures of 144/82 mm/Hg and 154/87 mm/Hg were achieved in the two groups, respectively. Fewer subjects in the tight control group had urine albumin concentration >50 mg/l than in the less tight control group at six years, although these differences were not significant at nine years of follow-up (21). Data from Action in Diabetes and vascular disease: Preterax and Diamicron Modified release controlled Evaluation (ADVANCE Trial) along with the African American Study of Kidney Disease and Hypertension (AASK) suggest that tight blood pressure control (<120/70 mmHg) in the context of diabetes and proteinuria improves kidney-specific outcomes. In the ADVANCE trial there were 11,140 enrolled subjects with T2DM who were randomly assigned to blood pressure treatment with fixed combination perindopril-indapamide or placebo. During the follow-up, mean systolic blood pressure (SBP) 134.7 and 140.3, and mean diastolic blood pressure (DBP) 74.8 and 77.0 mmHg was attained in the active treatment and placebo groups respectively. Active treatment not only decreased the risk for onset and progression of micro-albuminuria, it also increased the chance of regression of micro-albuminuria (20).
Over time evidence has accumulated to suggest renal benefits of tight blood pressure control in hypertensive subjects with diabetes, and has raised questions about treatment threshold. This question was addressed in Appropriate Blood pressure Control in Diabetics (Normotensive ABCD) study. Normotensive ABCD is a prospective randomized trial designed to study the effects of lowering blood pressure in normotensive (BP < 140/90 mm/Hg) subjects with diabetes. A total of 480 subjects were randomly assigned to intensive DBP control (target DBP of 10 mmHg below baseline) and moderate DBP control (target DBP 80-89 mmHg). The intensive treatment group was treated with nisoldipine or enalapril, and the moderate treatment group with placebo. Over a five year follow-up period, intensive blood pressure control (mean BP 128/75 mmHg) was associated with decreased risk for progression to incipient nephropathy and diabetic nephropathy in patients who were normotensive at baseline (22).
The importance of blood pressure control in patients with diabetes cannot be overemphasized. It is clear that blood pressure control is paramount for preservation of kidney function in subjects with diabetes especially as risk for progression to ESRD is increased up to seven-fold in patients with concomitant T2DM and hypertension (23).
Non-dipping blood pressure/pulse pattern in diabetes
A characteristic of diabetes includes a disproportionate elevation in SBP with a loss of nocturnal dipping of blood pressure and heart rate commonly referred to as “non-dipping” (24). In normo-tensive patients there is a circadian regulation of blood pressure wherein there are nocturnal drops in blood pressure roughly 10% to 15% commonly referred to as “dipping.” Alternatively, “non-dippers” have less than the usual 10% decline at night. Non-dipping is frequent among diabetic patients as demonstrated on ambulatory blood pressure monitoring. This “non-dipping” pattern is due, in part, to autonomic nervous system dysfunction often present in individuals with T2DM and characterized by a reduction in relative parasympathetic activity; it is thought to contribute to the five- to seven-fold increase in sudden death in diabetic patients (24, 25). Studies have shown that the non-dipping pattern of blood pressure is associated with micro-albuminuria, overt proteinuria, and higher morbidity and mortality in patients with diabetes (26). In this context, use of ambulatory blood pressure for measurement of dipping status is superior to office blood pressure in predicting target-organ involvement, such as proteinuria and left ventricular hypertrophy (24).
Blood pressure variability as a risk factor for DKD
There are a number of modifiable risk factors which predict development of incipient and overt kidney disease in people with obesity and diabetes (27, 28). Traditional risk factors for DKD include long term poor glycemic control, systemic and glomerular hypertension, hypercholesterolemia, urine albumin excretion rate (UAE), intrauterine growth retardation, and smoking (27-29). With regard to hypertension, attention has traditionally been focused on systolic, diastolic, and mean blood pressure with the assumption that conventional clinic readings depict a patient's true blood pressure and predict adverse outcomes (30). Blood pressure variability has been considered a random phenomenon of little clinical significance, although accumulating data suggest that visit to visit variability in blood pressure and episodic hypertension might affect cardiovascular and other target organ outcomes (30, 31). Emerging data also suggest that different drug classes affect blood pressure variability differently. Calcium channel blockers (CCB) and non-loop diuretics decrease blood pressure variability while beta blockers, angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) increase the blood pressure variability (32).
A post hoc analyses of diabetes control and complications trial (DCCT) demonstrate that a subject with SBP variability of 13.3 mmHg has a risk 2.34 times higher for kidney disease compared to a subject with variability of 3.7 mmHg (33). Observational data from a retrospective cohort study involving 354 patients with T2DM suggest that individuals who have greater visit to visit SBP variability might be at risk for development and progression of proteinuria (34). Recent data from multiple cohorts involving patients with previous transient ischemic attacks (TIA) and treated hypertension demonstrate a strong predictive value of visit to visit variability in SBP and maximum SBP for stroke and coronary events, independent of mean systolic pressures. Data from this study emphasize the risks of episodic hypertension, but do not prove a causal link between stroke and blood pressure variability or maximum systolic blood pressure (35). Data from a relatively small longitudinal retrospective observational study involving 374 elderly subjects with CKD showed association between visit to visit blood pressure variability and all-cause mortality. This study failed to show association between blood pressure variability and progression of CKD (36). Data accumulating from other studies points that visit to visit SBP variability might be associated with all-cause mortality and progression of vascular disease independent of mean arterial pressures in patients with or without diabetes (34-39).
Results of a meta-analysis suggest that variability of SBP between arms could be helpful for identification of people at increased risk for vascular disease (40). These findings have prompted investigators to study role of blood pressure difference between arms further and to explore its predictive value for other outcomes. Recently investigators studied the role of difference in SBP between arms and between lower limbs, in predicting risk for DKD. Initial data suggest that such blood pressure differences could be novel risk markers for DKD (41).
Accumulating data challenges the notion that mean arterial pressure or usual blood pressure is a sufficient predictor of vascular events, and stresses the need to analyze the available data and to explore the roles of other factors like blood pressure variability. Blood pressure variability is difficult to quantify and it is unclear how to incorporate it in the clinical practice. Further research is needed to better quantify associated risks and treatment parameters.
Use of (Angiotensin converting enzyme) ACE inhibitors and/or (Angiotensin receptor blockers) ARBs in DKD
The treatment of hypertension in those with DKD includes both non-pharmacologic and pharmacologic approaches. However, in the presence of DKD blood pressure reduction, use of pharmacologic strategies with interruption of the RAS with ACE inhibitors or ARBs is a primary risk reduction strategy (23, 42-45). Available data suggests that ARBs might have renal benefits independent of the SBP lowering effect in patients with T2DM (43, 44, 46). Data from a study that compared the reno-protective effects of telmisartan and enalapril suggest that ARBs and ACE inhibitors are equally effective in preventing loss of kidney function in subjects with T2DM and early DKD (47). Data from another large study show that losartan has significant beneficial effects on kidney function in patients with T2DM. Small differences in blood pressure were noted between the losartan and placebo treated groups, and it remains unclear to what extent the renal benefits in the group treated with losartan could be attributed to the lower blood pressure (46). Data from the Randomized Olmesartan And Diabetes MicroAlbuminuria Prevention (ROADMAP) trial also showed that the use of Olmesartan was associated with delay in onset of microalbuminuria but again there were subtle blood pressure differences between the two treatment groups (45).
However the benefit of dual RAS blockade has been in question. Data from the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET study) suggest that combined treatment with an ACE inhibitor and ARB was more effective than ACE inhibitor alone in reducing proteinuria, but the combination was associated with less desirable renal outcomes and faster decline in GFR (48). Available data suggest that individual components of RAS blockade help preserve kidney function better than other anti-hypertensives at least in people with proteinuria (48).
Effects of CKD on glucose homeostasis and assessment of glycemic control
Diabetes has been implicated in the development and progression of CKD, but progressive renal dysfunction also induces complex changes in insulin metabolism and clearance, and affects glucose homeostasis in patients with diabetes. CKD is associated with increased insulin resistance on one hand and decreased insulin clearance on other. A decrease in GFR is associated with decrease in metabolic clearance of insulin, which becomes exceedingly apparent as GFR falls below 15-20 ml/min/1.73m2. Usually as renal function declines, peritubular insulin uptake increases and maintains insulin clearance, but as GFR declines to levels below 15-20 ml/min/1.73m2, peritubular insulin uptake is unable to compensate for decreased renal function (49). With progression of CKD, the degradation of insulin in the liver and muscle is also impaired due to accumulation of the uremic byproducts. This decreased insulin clearance can decrease the insulin requirements in diabetes and can lead to hypoglycemic episodes. The decreased insulin clearance in CKD is counterbalanced by increased insulin resistance and decreased insulin production in patients with CKD (50). Many other factors like loss of appetite, malnutrition and deficient renal gluconeogenesis and catecholamine release impact glucose homeostasis in renal disease (50). Complex interactions of multiple divergent pathways make the determination of insulin requirement challenging in patients with DKD.
Lack of a standardized clinical test for monitoring glycemic control in DKD complicates management of diabetes in this patient subgroup. Glycated hemoglobin which is widely used to evaluate glycemic control in diabetes provides a retrospective assessment of glycemic control. HbA1c has been found to reliably access glycemic control in patients with diabetes, but its accuracy in patients with DKD is questionable. HbA1c levels are impacted by high urea levels, uremic acidosis, reduced red blood cell survival, and frequent blood transfusions, hence there is a potential for erroneous glycemic control estimates in patients with DKD (51).
Other markers of glycemic control such as glycated albumin and serum fructosamine assess glycemic control over two weeks, but these are unreliable in conditions affecting albumin metabolism (52). These tests have not been standardized and are not used frequently in clinical practice (53). Further studies are required to assess their use for diagnosis of diabetes and evaluation of glycemic control.
Markers of DKD and prognostic value of eGFR and microalbuminuria
Traditionally, eGFR and UAE have been used to define and to follow progression of DKD. In clinical practice, eGFR is estimated using clearance of endogenous creatinine. Release of creatinine into circulation is variable and depends on factors like age, gender, muscle mass, diet, volume status, medications, etc. Creatinine clearance further tends to overestimate GFR, due to tubular secretion of creatinine. Several equations like Cockcroft-Gault and Modification of Diet in Renal Disease four-variable (MDRD) have been used to improve the accuracy of GFR estimation, but these equations are less than perfect (2). Recently the Chronic Kidney Disease Epidemiological Collaboration) (CKD-EPI) equation was developed in an attempt to overcome the limitations of MDRD equation. CKD-EPI equation estimates GFR more accurately, especially at eGFR greater than 60 mL/min/1.73 m2 (54).
Other markers of GFR such as cystatin C have been studied, but have not received widespread acceptance in clinical practice due to associated costs. Limitations of currently available biomarkers for acute kidney injury (AKI) and CKD have prompted active interest in study of biomarkers. Many biomarkers are under investigation including; urinary podocytes, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), Smad-1, connective tissue growth factor (CTGF), and transforming growth factor beta (TGF-B) (2).
Along with eGFR, UAE is used to monitor progression and for staging of DKD. Although debatable, microalbuminuria is considered a risk predictor for progression to overt DKD and for CVD. Screening for microalbuminuria is widely recommended for risk stratification. Numerous population-based and intervention studies support microalbuminuria as a risk factor for CVD and as a strong predictor of cardiovascular morbidity and mortality in patients with diabetes (55, 56). Data from a study of 3431 diabetic subjects in the United Kingdom demonstrate that eGFR declined rapidly in people with macroalbuminuria and microalbuminuria, at rates of 5.7% and 1.5% per annum respectively. The progression of DKD was much slower in subjects with normoalbuminuria--eGFR decline of only 0.3% per year (57). Recently, a post hoc analysis of Nord-Trøndelag Health (HUNT-2) study showed that CKD progression risk increases substantially, in presence of micro or macroalbuminuria (58). Data from this analysis suggest a strong synergistic interaction between albuminuria and reduced eGFRwhich together confer much higher risk of progression to ESRD than attributable to either risk factor individually (58). This study highlights the importance of using UAE in combination with eGFR for better classification and risk stratification of patients with CKD (58).
It is clear that the risk for all cause and cardiovascular mortality increases with increase in UAE and decrease in eGFR. Data from a large retrospective study involving 1,120,295 adult subjects showed that low eGFR (≤60 mL/min/1.73m2) was independently associated with increased risk of death, cardiovascular events and hospitalization. The risks were substantially increased when eGFR decreased further to levels below 45 mL/min/1.73 m2. The adjusted hazard ratios for death were 1.2 (95% CI, 1.1 to 1.2), 1.8 (95% CI 1.7 to 1.9), 3.2 (95% CI , 3.1 to 3.4), and 5.9 ( 95% CI 5.4 to 6.5) for eGFR 45-59, 30-44, 15-29 and less than 15 respectively (17). Data from Renal Insufficiency And Cardiovascular Events (RIACE), a cross-sectional study involving 15,773 patients with T2DM led to similar conclusions. The data further showed that low eGFR and albuminuria ≥10.5 mg/24 hours are associated with coronary artery disease (CAD) in subjects with T2DM (59). A meta-analysis of albumin-to-creatinine ratio (ACR) data from over one million participants and urine protein dipstick data from 112,310 participants showed a significant increase in mortality risk at low eGFR (<60 mL/min/1.73 m2 or lower) compared with optimum eGFR (90-104 mL/min/1.73 m2 ) (6). Albuminuria was measured by ACR or urine dipstick in the included studies. The analyses showed that even trace protein on urine dipstick is associated with increased mortality in general population independent of eGFR and traditional cardiovascular risk factors (6). The hazard ratios for all-cause mortality were 1.20 (95% CI 1.15-1.26), 1.63 (1.50-1.77) and 2.22 (1.97-2.51) for ACR 1.1 mg/mmol, 3.4 mg/mmol and 33.9 mg/mmol respectively, compared to ACR of 0.6 mg/mmol (6). These findings highlight the importance of urine dipstick, an imprecise but inexpensive measure of albuminuria, in detection of DKD (6).
While some data would suggest CKD is not an independent risk factor for cardiovascular mortality (60, 61), many believe that CKD is independently associated with cardiovascular mortality and all-cause mortality (62-67). The confounding by prior CVD and by traditional and non-traditional CVD risk in patients with established CKD, makes data interpretation challenging (17). It is unclear if the increased cardiovascular mortality in CKD is an independent effect, or if it can be attributed to confounding factors. The role of other pathological changes like hypercoagulability, endothelial dysfunction, arterial stiffness, and increased inflammatory response as cardiovascular outcome modulators in people with CKD is an area of active interest (17).
Current literature supports the simultaneous use of eGFR and UAE for better risk stratification of patients with DKD. When used simultaneously, these markers help predict CKD progression and cardiovascular risk in patients with DKD.
DKD without albuminuria
Proteinuria has traditionally been considered a diagnostic and prognostic marker of DKD, and its presence prompts interventions such as initiation of ACE inhibitors or ARBs. Absence of proteinuria can render a false sense of reassurance for clinicians and often delays diagnosis and treatment of DKD. It is now evident that development and progression of microalbuminuria in subjects with DKD is not a rule and there is a distinct population that does not develop any level of proteinuria until late in disease. There is a possibility of stabilization and even regression of microalbumiuria in patients with diabetes (68). DKD is thought to arise from microvascular damage, which leads to increased UAE (69). But over time data have accumulated to suggest a high prevalence of kidney disease in patients with diabetes and normal UAE, suggesting the presence of renal lesions other than classic diabetic glomerulosclerosis in this population subgroup. This has prompted investigators to consider other explanations like interstitial fibrosis, ischemic vascular disease, cholesterol microemboli, atherosclerotic involvement of the renal vasculature, etc. (69, 70).
Recently researchers studied the development of nephropathy in the Cohen diabetic rat (an experimental model of human T2DM). The Cohen diabetic sensitive rats develop CKD with reduced eGFR and histological changes consistent with DKD as evidenced by light and electron microscopy, in absence of proteinuria, when fed diabetogenic diet. These rats develop changes suggestive of non-proliferative retinopathy as well, though these changes appeared later than development of DKD (71).
The characteristic histological lesions seen in classical diabetic glomerulosclerosis are often seen with other systemic manifestations of microvascular disease. These lesions include increased basement membrane thickness, diffuse mesangial sclerosis with nodular formation, hyalinosis, microaneurysm and hyaline arteriosclerosis (70, 71).
Data from the Developing Education on Microalbuminuria for Awareness of Renal and Cardiovascular Risk in Diabetes (DEMAND), a global cross-sectional study, showed that kidney dysfunction is not uncommon in T2DM with normal UAE (72). Kramer et al., had performed a cross-sectional analysis of a nationally representative sample of adults with T2DM and found that about 30% individuals with eGFR <60 did not have retinopathy or microalbuminuria (70). Data from other cross-sectional studies like RIACE, and longitudinal studies like atherosclerosis risk in communities (ARIC) and UKPDS suggest that normoalbuminuric renal impairment occurs frequently in subjects with T2DM (68, 73-75). Macroangiopathy could potentially be the underlying renal pathology as opposed to microangiopathy, in diabetic patients with normoalbuminuric CKD (73). This change in phenotype of DKD could be related to better control of risk factors like hyperglycemia, hyperlipidemia, hypertension, and early use of ACE inhibitors and ARBs (73).
Recent findings have encouraged investigators to think of microalbuminuria and reduced eGFR as markers of different pathological processes. Microalbumiuria could be phenotypic expression of endothelial dysfunction, while reduced eGFR could be renal manifestation of systemic atherosclerosis (67).
Does better glycemic control reduce DKD?
Data from Diabetes Control and Complications Trial (DCCT) and UKPDS have shaped the understanding and management of diabetes over the years for cardiovascular and kidney disease risk reduction. In UKPDS subjects with T2DM were randomly assigned to intensive or conventional glycemic control using insulin or oral hypoglycemic agents. Over 10 years, HbA1C 7% and 7.9% was achieved in the intensive and conventional groups respectively. The subjects assigned to intensive treatment protocols had decreased risk of micro-vascular complications, but the intensive treatment was associated with more hypoglycemic episodes and weight gain. The data also suggested that intensive control was associated with decreased progression of albuminuria (76). Post-trial monitoring of subjects enrolled in UKPDS, without any attempts to maintain previous diabetes therapies, showed an early loss (at one year) of glycemic differences between the two cohorts. Over a 10 year follow-up, a sustained benefit and continued risk reduction for microvascular complications, was observed in the cohort previously subject to intensive diabetes therapy (77).
Action in diabetes and vascular disease: Preterax and Diamicron modified release Controlled Evaluation (ADVANCE) is a multicenter RCT designed to study the effects of intensive glucose control (target HbA1c <6.5%) on vascular outcomes in T2DM. Mean HbA1c levels of 6.5% and 7.3% were achieved in the intensive and standard therapy groups respectively. Data from this trial showed significant reduction in the incidence of nephropathy with intensive glycemic control. Intensive treatment was also associated with decreased need for renal replacement therapy and death from complications related to kidney disease (78).
Previously data from DCCT showed beneficial effects of intensive vs. conventional glycemic control on kidney function in subjects with type 1 diabetes. Conventional therapy aimed at prevention of symptoms attributable to glycosuria or hyperglycemia and maintenance of normal growth and development, while intensive therapy aimed at achieving pre-prandial blood glucose 70-120 mg/dL and postprandial blood glucose concentration <180 mg/dL. Over a mean follow-up period of 6.5 years, microalbuminuria and albuminuria developed in fewer subjects on intensive treatment, compared to subjects on conventional treatment, leading to conclusions that intensive blood glucose management in subjects with insulin-dependent diabetes can delay the onset and slow the progression of diabetic nephropathy (79). The DCCT participants were followed in the Epidemiology of Diabetes Interventions and Complications (EDIC) study, an observational study post the DCCT closeout. The DCCT intensive treatment cohort was encouraged to continue the intensive treatment and the conventional treatment cohort was encouraged to switch to intensive treatment. Over eight years of further follow-up, the HbA1c difference between the two groups narrowed, with mean values of 8.0% and 8.2% in the two cohorts respectively. The incidence of microalbuminuria and clinical albuminuria was significantly lower in the group subject to intensive treatment during the DCCT trial (80). Further follow-up data have shown the extension of benefits of early intensive diabetes treatment in subjects with insulin-dependent diabetes for up to 22 years. The subjects in the intensive treatment arm of DCCT had a 50% lower risk of impaired GFR at 22 years of follow-up, compared to subjects in the conventional treatment arm, suggesting a “metabolic memory” effect (81). These data further suggest that intensive therapy of insulin-dependent diabetes in 29 subjects for 6.5 years can prevent impaired GFR in one subject over 20 years (82).
Data from these well-designed prospective trials indicate that better glycemic control has an important role in delaying the onset, and slowing the progression of nephropathy in patients with diabetes.
While good glycemic and blood pressure control remain the cornerstones of treatment strategy, to prevent or to slow progression of DKD, other treatment approaches are being explored. Recently effects of treatment with linagliptin, either alone or in combination with telmisartan, were studied in a mouse model of diabetic nephropathy. The combination seemed to have beneficial effects on albuminuria in mice, but its role in treatment or prevention of DKD in humans needs to be explored (83).
Pharmacological treatment of hyperglycemia in DKD/ use of old and new drugs other than insulin
CKD affects metabolism of oral hypoglycemic agents (OGA) and leads to accumulation of their metabolites, thus limiting the therapeutic options for patients with DKD. As discussed previously, renal dysfunction alters glucose homeostasis in unpredictable ways via multiple mechanisms in patients with DKD. This makes management of diabetes, especially glycemic control, challenging in DKD. The alterations in glucose and insulin handling by kidneys and other body tissues in DKD lead to a state of glycemic dysregulation, which is associated with increased risk of hypoglycemia as well as hyperglycemia (53).
Selection of an appropriate therapeutic modality is complicated by pharmacokinetic alterations caused by reduced kidney function (Table 1).
Table 1.
Dosage of drugs used to manage hyperglycemia in patients with diabetes and CKD/DKD. Adapted from (84, 86-90)
| Drug Class | Drug | Major Action | Dosing recommendation in CKD | Dosing recommendation in dialysis |
|---|---|---|---|---|
| Sulfonylureas | Glipizide | Insulin Scretagouge | No dose adjustment required | No dose adjustment required |
| Glimepiride | Insulin Scretagouge | Initiate at 1 mg/day and titrate slowly | Avoid | |
| Glyburide | Insulin scretagouge | Not recommended | Not recommended | |
| Alpha Glucosidase inhibitors | Acarbose | Slow carbohydrate absorption | Not recommended in sCr>2 mg/dL | Not recommended |
| Miglitol | Slow carbohydrate absorption | Not recommended if sCr> 2mg/dL | Not recommended | |
| -Meglitinides | Repaglinide | Insulin Scretagouge | Initiate at a lower dose 0.5mg before each meal if GFR< 40 | Use not studied |
| Nateglinide | Insulin scretagouge | Initiate at a low dose 60 mg before each meal | Avoid | |
| Biguanides | Metformin | Liver insulin sensitizer | Contraindicated if sCr >= 1.5 mg/dL in men, and >= 1.4 mg/dL in women | Not recommended |
| Thaizolidinediones | Rosiglitazone | Peripheral insulin sensitizer | No dose adjustment | No dose adjustment |
| Pioglitazone | Peripheral insulin sensitizer | No dose adjustment | No dose adjustment | |
| Incretin Minmetics | Exenatide | Improved insulin secretion | GFR > 50 no dose adjustment GFR 30-50 cautious use, but no dose adjustment suggested GFR < 30 use not recommended |
Use not recommended |
| Liraglutide | Improved insulin secretion | No dose adjustment | No dose adjustment | |
| DPP-4 inhibitors | Sitagliptin | Improved insulin secretion | GFR > 50 no dose adjustment, use 100mg/day GFR 30- 50 use 50 mg/day GFR < 30 use 25 mg/day |
Use 25 mg/day |
| Alogliptin | Improved insulin secretion | GFR > 50 no dose adjustment GFR 50-30 use 12.5 mg/day GFR < 30 use 6.25 mg/day |
Use 6.25 mg/day | |
| Linagliptin | Improved insulin secretion | No dose adjustment | No dose adjustment | |
| Saxagliptin | Improved insulin secretion | GFR> 50- no dose adjustment GFR< 50- use 2.5 mg/day |
Use 2.5 mg/day | |
| Amyin Analog | Pramlintide | Increased satiety and decreased glucagon | GFR > 20 no dose adjustment | Lacks clinical data |
| Sodium Glucose cotransporter 2 inhibitors | Canagliflozin | Glucuresis | GFR ≥ 60 No dose adjustment ( use 100- 300mg daily) GFR 45-60 (Maximum dose 100 mg/ day) GFR 30- 45 Use not recommended GFR < 30 Contraindicated |
Contraindicated |
Sulfonylureas
Sulfonylureas are insulin secretagogues and they increase endogenous insulin secretion. There is a high risk of hypoglycemia, especially with the use of longer acting sulfonylureas like glyburide (84).
Second generation sulfonylureas like Glipizide and Glimepiride can be used in patients with diabetes and CKD. Glyburide should be avoided due to its long half-life. Glimepiride should be initiated at a low dose in patients with CKD, and should be avoided in patients on dialysis.
Glipizide is the preferred second-generation sulfonylurea for patients with diabetes and CKD, and no dosage adjustment is required for patients with CKD or for those on dialysis (84).
Meglitinides
These are insulin secretagogues with rapid onset of action and short half-life. Repaglinide and nateglinide are the two meglitinides available in USA (85).
No dose adjustment is required while using repaglinide in patients with CKD or for those on dialysis, but it is recommended that repaglinide be initiated at a lower dose (0.5 mg before each meal) in patients with GFR <40 (84). Use in people with GFR <20 or those on dialysis has not been studied. Nateglinide should be initiated at a lower dose, (60mg before each meal) in patients with CKD and should be avoided in patients on dialysis (84).
Alpha-Glucosidase Inhibitors
These drugs prevent or decrease postprandial hyperglycemia. They work by decreasing the rate of breakdown of complex carbohydrates in the intestine, and thus decrease the amount of glucose available for absorption (86). Acarbose has minimal systemic absorption, but the drug and its metabolite tend to accumulate in patients with severe renal dysfunction. Similarly higher plasma levels of miglitol are present in patients with severe renal failure compared to patients with normal renal function, when on equal doses of miglitol. Acarbose and miglitol are available in the US, but are not recommended for patients with serum creatinine >2mg/dl or on dialysis, as long-term safety of these drugs in patients with CKD has not been studied (84).
Biguanides
Metformin suppresses gluconeogenesis by decreasing hepatic insulin resistance. It effectively decreases glucose concentration in fasting as well as postprandial states (85).
Metformin should be avoided in patients with moderate and severe renal failure, as renal clearance of metformin is decreased in patients with renal impairment, leading to accumulation of the drug, and increased risk of lactic acidosis. Its use in contraindicated in men with sCr >= 1.5mg/dL and women with sCr >= 1.4 mg/dL (84).
Thiazolidinedione
Thiazolidinediones are agonist of peroxisome proliferator-activated receptor gamma. The stimulation of this receptor increases insulin stimulated glucose uptake in muscles and adipose tissue, and decreases hepatic glucose production and insulin resistance (85).
Rosiglitazone and pioglitazone are available in the US and these can be used in patients with CKD without dose adjustment. However, these drugs should be used with caution in those with advanced CKD due to concerns of volume retention. Careful attention should be given to volume status of patients as thiazolidinediones can cause fluid retention, hemodilution and exacerbation of heart failure (84).
Incretin Mimetics
Glucagon like peptide-1 (GLP-1) is an incretin which increases glucose dependent insulin secretion. It also slows gastric emptying, increases satiety, and thus decreases food intake (85). Exenatide and Liraglutide are the GLP-1 analogues available in the US. Use of an exenatide is not recommended in patients with CrCl < 30 mL/min, or in those on dialysis (84). Close monitoring is required while initiating or up-titrating the dose of exenatide, especially in patients with mild to moderate renal dysfunction, as use of exenatide is associated with nausea and vomiting, and potential for volume depletion and worsening of renal function. No renal dose adjustment is required for liraglutide, and it can be used safely in patients with CKD or ESRD, though attention to volume status is warranted due to associated nausea and vomiting (84).
Dipeptidylpeptidase-4 (DPP-4) Inhibitors
DPP-4 inhibitors inhibit DPP-4 and thus prevent degradation of GLP-1. Sitagliptin, linagliptin, saxagliptin and alogliptin are the DPP-4 inhibitors available in the US.The dose of sitagliptin and alogliptin needs to be decreased by 50% and 75% for GFR 50-30 mL/min/1.73m2 and <30 mL/min/1.73 m2 respectively (84). Saxagliptin can be dosed at 2.5 to 5 mg daily if the GFR >50, but for patients with lower GFR or ESRD, dose of 2.5 mg/day should be used (87). No dosage adjustment is required in patients with CKD for linagliptin (88).
Amylin Analogue
Amylin is secreted along with insulin by pancreatic beta cells. Pramlintide is a synthetic analogue of amylin, and pre-prandial administration of pramlintide is associated with decreased plasma glucagon, slower gastric emptying and increased satiety (85). This medication is metabolized primary in the kidney, but no change in dose is required if the creatinine clearance is more than 20 ml/min/1.73 m2. Data are lacking to recommend use of pramlintide in patients on dialysis (84).
Sodium Glucose Cotransporter 2 (SGLT2) Inhibitors
SGLT2 inhibitors decrease renal threshold for glucose and, induce glucuresis independent of insulin action. These agents induce renal excretion of glucose and have the potential to cause weight loss, by disposing excess calories/glucose (89). Canagliflozin is a SGLT2 inhibitor that has been recently approved in the US. Its efficacy in subjects with diabetes and stage 3 CKD (eGFR ≥ 30 and <50 ml/min/1.73 m2) has been demonstrated in a placebo controlled RCT (90). Efficacy of canagliflozin is dependent on renal function and this drug is not expected to be effective in subjects with eGFR < 30 ml/min/1.73 m2 or in those on dialysis (90). SGLT2 inhibitors have an osmotic diuretic effect and can lead to plasma volume depletion, so kidney function should be monitored while initiating this drug in subjects with DKD.
Many new therapeutic agents have been introduced for treatment of diabetes in patients with or without CKD, but special attention to renal function is warranted when choosing the appropriate agent and dose adjustments should be made to prevent any deleterious effects.
Conclusions
Diabetes is increasingly prevalent and is an important cause of CKD and ESRD. Recently attention has been focused on DKD without albuminuria and its pathogenesis is being studied. There are some indications that pathogenesis of diabetic nephropathy, in the absence of albuminuria, might differ from that of traditional diabetic nephropathy with micro-albuminuria. Review of recent trial data indicates that better glycemic and blood pressure control can delay the onset and slow the progression of kidney disease in patients with diabetes. Use of several older oral hypoglycemic agents is either contraindicated or requires dosage adjustment in CKD. New medications for diabetes have been approved recently and many can be used safely in patients with CKD, thus providing treatment alternatives for better glycemic control in patients who are reluctant to use insulin. We further suggest that DKD should be considered in a much broader context of cardiorenal metabolic syndrome rather than just diabetes, and close attention should be paid to other modifiable cardiorenal risk factors.
Key points.
Diabetic nephropathy should be studied and treated in broad context of cardiorenal syndrome, with a focus on the complex intertwined metabolic changes, which increase CKD and CVD risk.
Blood pressure and glycemic control are crucial for prevention and treatment of DKD.
Newer drugs for achieving glycemic control have an important role in the treatment of T2DM in patients with cardiorenal syndrome.
Acknowledgments
Disclosures:
Funding Sources:
Dr. Sowers: NIH (R01 HL73101-01A1 & R01 HL107910-01) Veterans Affairs Merit System 0019
Dr. Whaley-Connell: NIH (R-03 AG040638) Veterans Affairs System (CDA-2) ASN-ASP Junior Development Grant in Geriatric Nephrology, supported by a T. Franklin Williams Scholarship Award
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Dr. Sowers: Merck Pharmaceuticals Advisory Board
bibliography
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA : the journal of the American Medical Association. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Reeves WB, Rawal BB, Abdel-Rahman EM, et al. Therapeutic Modalities in Diabetic Nephropathy: Future Approaches. Open J Nephrol. 2012;2:5–18. doi: 10.4236/ojneph.2012.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes care. 2004;27:S79–83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 4.Whaley-Connell A, Chaudhary K, Misra M, et al. A Case for Early Screening for Diabetic Kidney Disease. Cardiorenal Med. 2011;1:235–42. doi: 10.1159/000332386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Chronic Kidney Disease Fact Sheet 2010. 2010 [Google Scholar]
- 6.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sowers JR. Metabolic risk factors and renal disease. Kidney Int. 2007;71:719–20. doi: 10.1038/sj.ki.5002006. [DOI] [PubMed] [Google Scholar]
- 8.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2013;124:139–52. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 9.Najafian B, Alpers CE, Fogo AB. Pathology of human diabetic nephropathy. Contrib Nephrol. 2011;170:36–47. doi: 10.1159/000324942. [DOI] [PubMed] [Google Scholar]
- 10.Tervaert TW, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–63. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 11.Sowers JR, Whaley-Connell A, Hayden MR. The Role of Overweight and Obesity in the Cardiorenal Syndrome. Cardiorenal Med. 2011;1:5–12. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jindal A, Brietzke S, Sowers JR. Obesity and the Cardiorenal Metabolic Syndrome: Therapeutic Modalities and Their Efficacy in Improving Cardiovascular and Renal Risk Factors. Cardiorenal Med. 2012;2:314–27. doi: 10.1159/000343803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakris G, Vassalotti J, Ritz E, et al. National Kidney Foundation consensus conference on cardiovascular and kidney diseases and diabetes risk: an integrated therapeutic approach to reduce events. Kidney Int. 2010;78:726–36. doi: 10.1038/ki.2010.292. [DOI] [PubMed] [Google Scholar]
- 14.Whaley-Connell A, Pavey BS, Afroze A, et al. Obesity and insulin resistance as risk factors for chronic kidney disease. J Cardiometab Syndr. 2006;1:209–14. doi: 10.1111/j.1559-4564.2006.05631.x. [DOI] [PubMed] [Google Scholar]
- 15.U.S.Renal Data System . USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD.: 2010. [Google Scholar]
- 16.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. The New England journal of medicine. 2004;351:1285–95. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 19.Jindal A, Connell AW, Sowers JR. Type 2 Diabetes in older people; The importance of blood pressure control. Current cardiovascular risk reports. 2013 doi: 10.1007/s12170-013-0301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Galan BE, Perkovic V, Ninomiya T, et al. Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol. 2009;20:883–92. doi: 10.1681/ASN.2008070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Bmj. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 22.Schrier RW, Estacio RO, Esler A, et al. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–97. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 23.Ruggenenti P, Perna A, Ganeva M, et al. Impact of blood pressure control and angiotensin-converting enzyme inhibitor therapy on new-onset microalbuminuria in type 2 diabetes: a post hoc analysis of the BENEDICT trial. J Am Soc Nephrol. 2006;17:3472–81. doi: 10.1681/ASN.2006060560. [DOI] [PubMed] [Google Scholar]
- 24.Pickering TG, Kario K. Nocturnal non-dipping: what does it augur? Curr Opin Nephrol Hypertens. 2001;10:611–6. doi: 10.1097/00041552-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen FS, Hansen HP, Jacobsen P, et al. Increased sympathetic activity during sleep and nocturnal hypertension in Type 2 diabetic patients with diabetic nephropathy. Diabetic medicine : a journal of the British Diabetic Association. 1999;16:555–62. doi: 10.1046/j.1464-5491.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 26.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. Journal of hypertension. 2002;20:2183–9. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes care. 2002;25:859–64. doi: 10.2337/diacare.25.5.859. [DOI] [PubMed] [Google Scholar]
- 28.Gall MA, Hougaard P, Borch-Johnsen K, et al. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. Bmj. 1997;314:783–8. doi: 10.1136/bmj.314.7083.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parving HH. Renoprotection in diabetes: genetic and non-genetic risk factors and treatment. Diabetologia. 1998;41:745–59. doi: 10.1007/s001250050983. [DOI] [PubMed] [Google Scholar]
- 30.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–48. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 31.Rossignol P, Kessler M, Zannad F. Visit-to-visit blood pressure variability and risk for progression of cardiovascular and renal diseases. Curr Opin Nephrol Hypertens. 2013;22:59–64. doi: 10.1097/MNH.0b013e32835b489f. [DOI] [PubMed] [Google Scholar]
- 32.Webb AJ, Fischer U, Mehta Z, et al. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–15. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 33.Kilpatrick ES, Rigby AS, Atkin SL. The role of blood pressure variability in the development of nephropathy in type 1 diabetes. Diabetes care. 2010;33:2442–7. doi: 10.2337/dc10-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okada H, Fukui M, Tanaka M, et al. Visit-to-Visit Blood Pressure Variability Is a Novel Risk Factor for the Development and Progression of Diabetic Nephropathy in Patients With Type 2 Diabetes. Diabetes care. 2013;22:22. doi: 10.2337/dc12-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 36.Di Iorio B, Pota A, Sirico ML, et al. Blood pressure variability and outcomes in chronic kidney disease. Nephrol Dial Transplant. 2012;27:4404–10. doi: 10.1093/ndt/gfs328. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh YT, Tu ST, Cho TJ, et al. Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: a 5.5-year prospective analysis. Eur J Clin Invest. 2012;42:245–53. doi: 10.1111/j.1365-2362.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 38.Okada H, Fukui M, Tanaka M, et al. Visit-to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2012;220:155–9. doi: 10.1016/j.atherosclerosis.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Muntner P, Shimbo D, Tonelli M, et al. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–6. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 40.Clark CE, Taylor RS, Shore AC, et al. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012;379:905–14. doi: 10.1016/S0140-6736(11)61710-8. [DOI] [PubMed] [Google Scholar]
- 41.Okada H, Fukui M, Tanaka M, et al. A difference in systolic blood pressure between arms and between lower limbs is a novel risk marker for diabetic nephropathy in patients with Type 2 diabetes. Hypertens Res. 2013;17:207. doi: 10.1038/hr.2012.207. [DOI] [PubMed] [Google Scholar]
- 42.Ravid M, Brosh D, Levi Z, et al. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Annals of internal medicine. 1998;128:982–8. doi: 10.7326/0003-4819-128-12_part_1-199806150-00004. [DOI] [PubMed] [Google Scholar]
- 43.Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. The New England journal of medicine. 2001;345:870–8. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 44.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. The New England journal of medicine. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 45.Haller H, Ito S, Izzo JL, Jr., et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. The New England journal of medicine. 2011;364:907–17. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 46.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. The New England journal of medicine. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 47.Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. The New England journal of medicine. 2004;351:1952–61. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 48.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–53. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 49.Mak RH. Impact of end-stage renal disease and dialysis on glycemic control. Semin Dial. Jan-Feb. 2000;13(1):4–8. doi: 10.1046/j.1525-139x.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 50.Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial. 2010;23:148–56. doi: 10.1111/j.1525-139X.2010.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ansari A, Thomas S, Goldsmith D. Assessing glycemic control in patients with diabetes and end-stage renal failure. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2003;41:523–31. doi: 10.1053/ajkd.2003.50114. [DOI] [PubMed] [Google Scholar]
- 52.Koga M, Murai J, Saito H, et al. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes care. 2010;33:270–2. doi: 10.2337/dc09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kovesdy CP, Sharma K, Kalantar-Zadeh K. Glycemic control in diabetic CKD patients: where do we stand? American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;52:766–77. doi: 10.1053/j.ajkd.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Archives of internal medicine. 1997;157:1413–8. [PubMed] [Google Scholar]
- 56.Jensen T, Borch-Johnsen K, Kofoed-Enevoldsen A, et al. Coronary heart disease in young type 1 (insulin-dependent) diabetic patients with and without diabetic nephropathy: incidence and risk factors. Diabetologia. 1987;30:144–8. doi: 10.1007/BF00274218. [DOI] [PubMed] [Google Scholar]
- 57.Hoefield RA, Kalra PA, Baker PG, et al. The use of eGFR and ACR to predict decline in renal function in people with diabetes. Nephrol Dial Transplant. 2011;26:887–92. doi: 10.1093/ndt/gfq526. [DOI] [PubMed] [Google Scholar]
- 58.Hallan SI, Ritz E, Lydersen S, et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–77. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solini A, Penno G, Bonora E, et al. Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes care. 2012;35:143–9. doi: 10.2337/dc11-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garg AX, Clark WF, Haynes RB, et al. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61:1486–94. doi: 10.1046/j.1523-1755.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- 61.Culleton BF, Larson MG, Wilson PW, et al. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–9. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 62.Drey N, Roderick P, Mullee M, et al. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2003;42:677–84. doi: 10.1016/s0272-6386(03)00916-8. [DOI] [PubMed] [Google Scholar]
- 63.Muntner P, He J, Hamm L, et al. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–53. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura K, Okamura T, Hayakawa T, et al. Chronic kidney disease is a risk factor for cardiovascular death in a community-based population in Japan: NIPPON DATA90. Circ J. 2006;70:954–9. doi: 10.1253/circj.70.954. [DOI] [PubMed] [Google Scholar]
- 65.Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63:1121–9. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 66.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 67.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–21. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Retnakaran R, Cull CA, Thorne KI, et al. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832–9. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 69.MacIsaac RJ, Panagiotopoulos S, McNeil KJ, et al. Is nonalbuminuric renal insufficiency in type 2 diabetes related to an increase in intrarenal vascular disease? Diabetes care. 2006;29:1560–6. doi: 10.2337/dc05-1788. [DOI] [PubMed] [Google Scholar]
- 70.Kramer HJ, Nguyen QD, Curhan G, et al. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA : the journal of the American Medical Association. 2003;289:3273–7. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 71.Yagil C, Barak A, Ben-Dor D, et al. Nonproteinuric diabetes-associated nephropathy in the Cohen rat model of type 2 diabetes. Diabetes. 2005;54:1487–96. doi: 10.2337/diabetes.54.5.1487. [DOI] [PubMed] [Google Scholar]
- 72.Dwyer JP, Parving HH, Hunsicker LG, et al. Renal Dysfunction in the Presence of Normoalbuminuria in Type 2 Diabetes: Results from the DEMAND Study. Cardiorenal Med. 2012;2:1–10. doi: 10.1159/000333249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Penno G, Solini A, Bonora E, et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. Journal of hypertension. 2011;29:1802–9. doi: 10.1097/HJH.0b013e3283495cd6. [DOI] [PubMed] [Google Scholar]
- 74.Bash LD, Selvin E, Steffes M, et al. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Archives of internal medicine. 2008;168:2440–7. doi: 10.1001/archinte.168.22.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, et al. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes care. 2004;27:195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]
- 76.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 77.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. The New England journal of medicine. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 78.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. The New England journal of medicine. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 79.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. The New England journal of medicine. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 80.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA : the journal of the American Medical Association. 2003;290:2159–67. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Boer IH, Sun W, Cleary PA, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. The New England journal of medicine. 2011;365:2366–76. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Boer IH, Rue TC, Cleary PA, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Archives of internal medicine. 2011;171:412–20. doi: 10.1001/archinternmed.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alter ML, Ott IM, von Websky K, et al. DPP-4 inhibition on top of angiotensin receptor blockade offers a new therapeutic approach for diabetic nephropathy. Kidney Blood Press Res. 2012;36:119–30. doi: 10.1159/000341487. [DOI] [PubMed] [Google Scholar]
- 84.Abe M, Okada K, Soma M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice. Curr Drug Metab. 2011;12:57–69. doi: 10.2174/138920011794520053. [DOI] [PubMed] [Google Scholar]
- 85.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15:540–59. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]
- 86.Hanefeld M, Schaper F. Acarbose: oral anti-diabetes drug with additional cardiovascular benefits. Expert Rev Cardiovasc Ther. 2008;6:153–63. doi: 10.1586/14779072.6.2.153. [DOI] [PubMed] [Google Scholar]
- 87.Boulton DW, Li L, Frevert EU, et al. Influence of renal or hepatic impairment on the pharmacokinetics of saxagliptin. Clin Pharmacokinet. 2011;50:253–65. doi: 10.2165/11584350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 88.Friedrich C, Emser A, Woerle HJ, et al. Renal Impairment Has No Clinically Relevant Effect on the Long-Term Exposure of Linagliptin in Patients With Type 2 Diabetes. Am J Ther. 2013;13:13. doi: 10.1097/MJT.0b013e31826232dc. [DOI] [PubMed] [Google Scholar]
- 89.Whaley JM, Tirmenstein M, Reilly TP, et al. Targeting the kidney and glucose excretion with dapagliflozin: preclinical and clinical evidence for SGLT2 inhibition as a new option for treatment of type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2012;5:135–48. doi: 10.2147/DMSO.S22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes, obesity & metabolism. 2013;15:463–73. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. The Journal of pediatrics. 2009;155:049. doi: 10.1016/j.jpeds.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]