Figure 2.
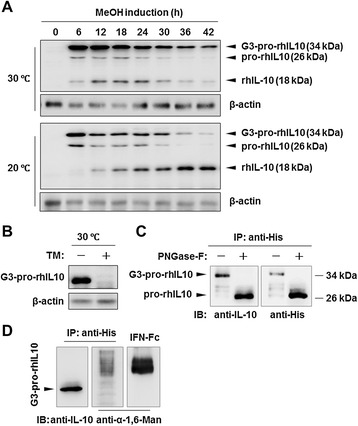
High-temperature cultivation of an rhIL-10 expression strain impairs the maturation of G3-pro-rhIL10. (A) Whole-cell lysates were prepared from cells as in Figure 1B and examined by Western blotting using an anti-His antibody. β-actin was used as a loading control. The bands labeled G3-pro-rhIL10, pro-rhIL10 and rhIL-10 represent the intracellular forms of rhIL-10. (B) The rhIL-10 expression strain (clone ‘H’) was methanol-induced at 30°C for 24 hours with or without the addition of 5 μM tunicamycin (TM) in shaking flask culture. Whole-cell lysates were examined by Western blotting. (C) Intracellular G3-pro-rhIL10 (34 kDa) was initially purified with Ni-affinity beads, de-glycosylated with PNGase F, and finally examined by Western blotting using anti-IL-10 and anti-His antibodies. (D) Purified G3-pro-rhIL10 was directly examined by Western blotting with anti-IL-10 and anti-α-1,6-mannose antibodies, and IFN-Fc produced from Pichia pastoris (made in our lab) was employed as a positive control. All the experiments were repeated three times and showed similar results.
