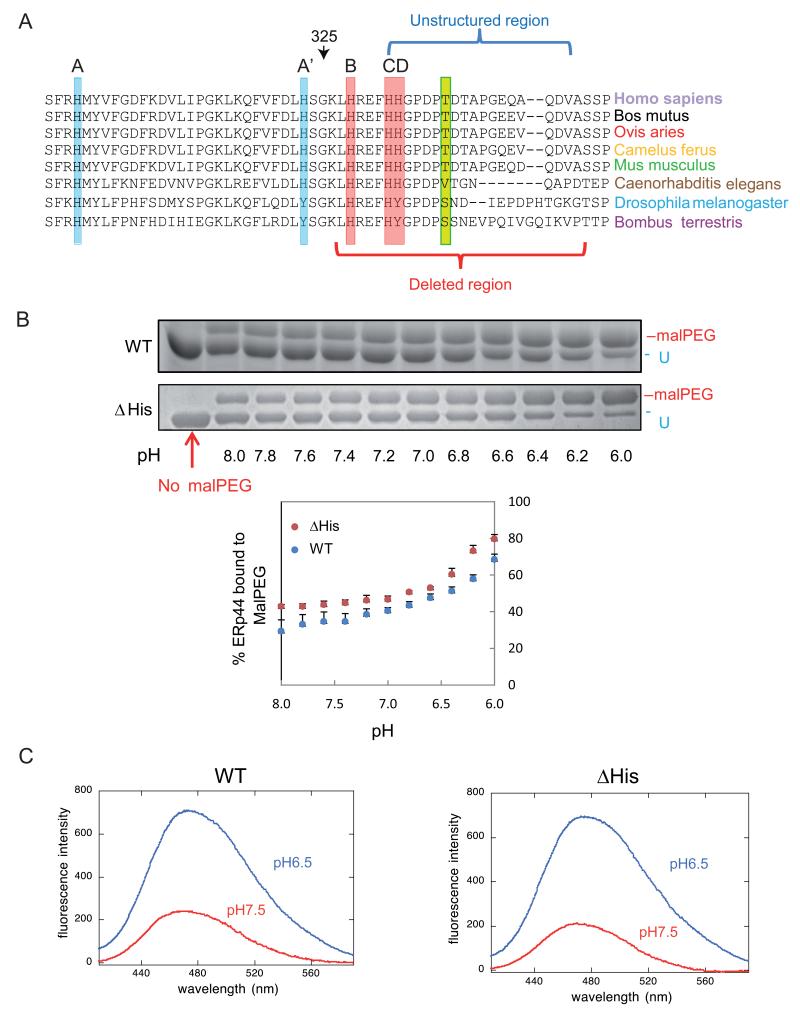
Figure 1. pH dependent conformational changes of ΔHis ERp44 in vitro.
A) Sequence conservation in the proximal part of the ERp44 C-tail. ClustalW2 alignment of the histidine-rich region and of the first part of the C-terminal tail of ERp44 reveals high sequence conservation in different organisms. Three histidines reside in the tail (B, C and D) and two in the b′ domain (A and A′). A, B and C (highlighted in red) are the most conserved histidines, while A′ and D (in pale blue) are absent in arthropods. The unstructured region in the crystal and the region deleted in the ΔHis mutant are indicated by blue and red brackets.
B) pH-dependent accessibility of ERp44 cysteine 29. Upon in vitro alkylation with MalPEG (10 minutes at room temperature), ERp44 undergoes an easily detectable mobility shift in SDS-PAGE, indicating the accessibility of C29 to MalPEG (upper two panels; (Vavassori et al., 2013)). The percentage of MalPEG-bound ERp44 was plotted as a function of pH (lower panel). Clearly, lowering the pH increases the accessibility of C29 to MalPEG in the ΔHis mutant as well as in wt ERp44.
C) pH-dependent tail movements in wt and ΔHis ERp44 compared by ANS binding in vitro. ANS fluorescence spectra were measured for ERp44 wt or ΔHis. The exposure of hydrophobic surfaces correlates with increased and blue-shifted ANS fluorescence (Vavassori et al., 2013). Also with this assay, ERp44 wt and ΔHis show similar pH dependent changes.