Figure 6.
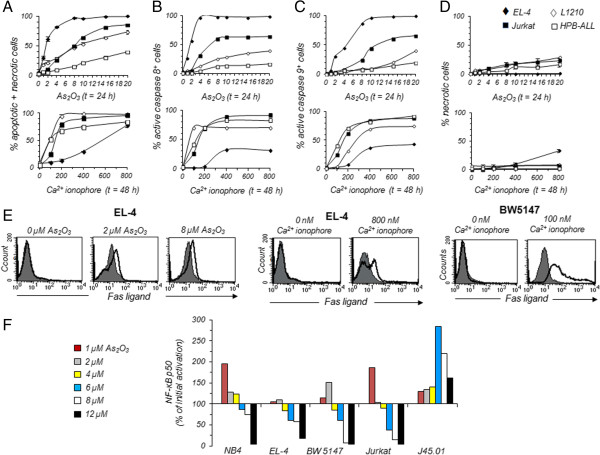
Contribution of apoptosis, necrosis and NF-κB p50 pathways in the cytotoxicity of As 2 O 3 or calcium ionophore A23187. (A to E) EL-4 (♦), L1210 (◊), Jurkat (■) and HPB-ALL (□) T-cell lines were treated with 1 to 20 μM As2O3 for 24 h or 100 to 800 nM A23187 for 48 h. Cells treated with As2O3 or A23187, or left untreated, were subsequently stained with PI and CaspaTag Caspase 8 or Caspase 9 fluorescein in situ fluorescence assay kit. The stained cells were analyzed by flow cytometry in order to determine the percentages of dead cells (either apoptotic or necrotic cells, graphs A) (n = 5 independent experiments), the percentages of PI+ apoptotic cells containing active caspase 8 (graphs B) or active caspase 9 (graphs C), the percentage of necrotic cells (graphs D). Human or murine FasL expression was detected using anti-FasL mAb and flow cytometry (histograms E). Histograms obtained with PE-conjugated Armenian hamster (clone MFL3) or mouse (clone NOK-1) anti-FasL mAb (open histogram) are overlaid on histograms obtained with PE-conjugated Armenian hamster or mouse IgG1 isotype control (shaded histogram) (n = 3 independent experiments). At least 20,000 events were analyzed for each sample. (F). Nuclear extracts were prepared from NB4, EL-4, BW5147, Jurkat and J45.01 cells treated with 1 to 12 μM As2O3 for 24 h. Levels of NF-κB p50 in 5 μg of nuclear extracts were quantified by a DNA binding ELISA assay. Results are representative of two other experiments.
