Abstract
Objective
Overweight and obese individuals, who comprise approximately two-thirds of the U.S. population, are at increased risk for developing a range of diseases. This increased risk may be due in part to maladaptive stress responses within this group, including heightened low-grade inflammation and HPA axis non-habituation. In this study we tested the relationship between adiposity, plasma interleukin-6 (IL-6) and HPA axis responses to repeated stress.
Methods
Sixty-seven healthy participants were exposed to the Trier Social Stress Test (TSST) on two consecutive days. We collected saliva for cortisol measurements at baseline and at 1, 10, 30, 60 and 120 minutes post-TSST, and blood for plasma IL-6 measurements at baseline and 30 and 120 minutes post-TSST.
Results
Stress exposure induced significant increases of cortisol and IL-6 on both days (cortisol: F=38, p<0.001; IL-6: F=90.8; p<0.001), and repeated exposure was related with cortisol habituation (F=8.2; p<0.001) and IL-6 sensitization (F=5.2; p=0.022). BMI and body fat were related with higher cortisol responses to repeated stress (BMI: beta=0.34; p=0.014; body fat: beta=0.29; p=0.045), and with higher IL-6 responses to repeated stress (BMI: beta=0.27, p=0.044; body fat: beta=0.37; p=0.006).
Conclusions
Taken together, individuals with higher measures of adiposity showed less efficient HPA axis habituation as well as sensitization of IL-6 responses to repeated acute stress. These findings point to maladaptive stress response patterns in overweight humans, which, through exposure to higher levels of inflammatory mediators, might partially explain diseases related with overweight and/or obesity.
Keywords: inflammation, obesity, adiposity, stress, IL-6, sensitization, TSST
2 Introduction
Approximately two thirds of Americans are overweight or obese, and worldwide obesity has almost doubled since 1980 (WHO 2013). Obesity is a major risk factor for many conditions including hyperlipidemia, hypertension, heart disease, stroke and Type II Diabetes (Lapidus, Bengtsson et al. 1984, Kissebah and Krakower 1994, Grundy 2002, Lavie, Milani et al. 2009). Adipose tissue is recognized as an endocrine organ capable of regulating metabolic function as well as secreting signaling molecules and cytokines (Trayhurn 2005). Chronic low-grade inflammation is a hallmark of obesity, and the dysregulated inflammation seen in obesity contributes to the pathology of a number of co-morbid conditions, including atherosclerosis, type 2 diabetes and fatty liver disease (Danesh, Collins et al. 1998, Danesh 1999, Black and Garbutt 2002). In addition, obesity-related inflammation is emerging as a mechanism for increased cancer risk (Roberts, Dive et al. 2010).
While there is strong evidence supporting the association of obesity with basal levels of inflammation (Rexrode, Pradhan et al. 2003, Panagiotakos, Pitsavos et al. 2005, Himmerich, Fulda et al. 2006, Thorand, Baumert et al. 2006, Brydon 2011), very little is known about how obesity affects psychosocial stress-induced increases of IL-6 concentrations. Among normal weight individuals, acute psychosocial stress induces an increase in plasma inflammatory molecules such as IL-6 (Steptoe, Hamer et al. 2007), which does not typically habituate to repeated stress. Therefore, recurrent psychosocial stressors result in repeated exposure to increased IL-6 (von Kanel, Kudielka et al. 2006, Rohleder 2014). Due to its relatively slow response and recovery, and its failure to habituate to repeated stressors, it has been suggested that low-grade peripheral inflammation up-regulated by psychosocial stress exposes individuals to sustained higher concentrations of inflammatory mediators over time, which in turn may increase disease risk (Rohleder 2014). This risk might be even more exaggerated in overweight and obese individuals because of the relationship between adipose tissue and inflammation, as well as increased basal IL-6 levels seen in overweight and obese individuals (Rexrode, Pradhan et al. 2003, Panagiotakos, Pitsavos et al. 2005, Himmerich, Fulda et al. 2006, Thorand, Baumert et al. 2006, Brydon 2011). One study examined the effect of central adiposity on response to a single mild psychological stressor in young women and found that while waist circumference was related to baseline IL-6, there was no relationship between adiposity and IL-6 response to stressor (Brydon 2011). However, this study was done in a sample limited to young women, with a mild psychological stressor administered only once. It is possible that differences in stress responses due to adiposity emerge with a more robust stressor administered repeatedly.
In addition to its relationship with chronic inflammation, there is evidence that obesity modulates the glucocorticoid response to stress, although the literature is inconsistent with regard to the directionality of associations. Obesity has been found to be associated with elevated baseline cortisol secretion and higher HPA-axis reactivity to psychological stress as well as physiological and pharmacological stimulation (Bjorntorp 1993). In line with these earlier findings, greater cortisol responses to stress have recently been noted in overweight women compared to leaner women (Benson, Arck et al. 2009). However, Jones et al. (2012) using a novel, magnetic resonance (MR) imaging-based method of body fat quantification found lower cortisol stress responses in individuals with adiposity (Jones et al., 2012). Only one study has addressed HPA axis habituation to repeated stress. Individuals with a high Waist-to-Hip Ratio (WHR), indicative of increased central adiposity, showed less efficient cortisol habituation in response to repeated stress compared to lean individuals with a low WHR (Epel, McEwen et al. 2000). Taken together, the relationships of measures of adiposity with cortisol responses to psychosocial stressors are documented in a limited number of studies and findings are inconsistent.
To our knowledge, there have been no studies reporting the effect of repeated psychosocial stress on low-grade inflammation in overweight individuals. In the present study we therefore aimed to examine whether measures of adiposity, including Body Mass Index (BMI), body fat percentage, waist circumference and waist to hip ratio, were associated with altered IL-6 and cortisol responses to repeated stress. We hypothesized that greater adiposity will be associated with increased IL-6 responses as well as altered cortisol responses, including less habituation to repeated stress. We also expected to replicate previous findings of higher baseline IL-6 and cortisol in overweight individuals.
3 Methods
3.1 Participants
Data were collected as part of a larger research project conducted over two years to investigate the effects of stress on endocrine and inflammatory parameters. Young adults (age 18–35 yrs.) and older adults (age 50–65 yrs.) were recruited from the Greater Boston area and the Brandeis University campus via newspaper, magazine, and Facebook advertisements. All participants underwent a brief medical and psychological screening by telephone before testing and were invited to participate only if they met the following selection criteria: (a) body mass index (BMI) within the reference range between 18 and 35 kg/m2; (b) luteal phase of menstrual cycle at time of participation, for females; (c) absence of psychiatric, endocrine, or cardiovascular diseases, or other specific chronic diseases; (d) no intake of psychoactive drugs, beta-blockers, gonadal steroids (hormonal contraceptives), GCs; (e) non-smoker, and (f) no previous experience with the stress protocol. Individuals were paid for their participation.
We recruited n=72 individuals for participation in this study. Five participants were excluded from IL-6 analyses because their IL-6 baseline or responses were greater than 2.5 standard deviations above the mean. In addition, one participant discontinued after the first session, and two participants displayed signs of infection during session two, and therefore only their biological data from session one was used. This left a final sample of n=67 for day 1 IL-6 and n=64 for day 2 IL-6 analyses. N=6 participants included in the IL-6 analysis were excluded from cortisol analyses because they did not have cortisol data. N=9 participants were excluded because their baseline cortisol was over 15 nmol/l. This left a final sample of n=56 participants for day 1 and 2 cortisol analyses. Two participants were missing body fat percentage data.
3.2 Procedure
Eligible participants were scheduled for laboratory sessions on two consecutive days. All laboratory sessions were scheduled in the afternoon (13:30–18:30 h) to control for circadian variation of cortisol, and participants came in at the same time for both sessions. Participants were instructed to refrain from eating or drinking anything but water for one hour before the laboratory sessions. Written informed consent was obtained prior to participation. The Brandeis University Institutional Review Board approved all procedures.
Each laboratory session lasted approximately three hours and included a 30-minute resting period followed by exposure to the Trier Social Stress Test (TSST; Kirschbaum et al., 1993) Saliva samples for measurement of free cortisol were collected using Salivette collection devices (Sarstedt, Newton, NC) at baseline, as well as 1, 10, 30, 60 and 120 minutes post TSST on both study days. Details on the saliva collection procedure are included below.
For assessment of IL-6 concentrations, blood was drawn via the antecubital vein using a peripheral venous catheter (BD Nexiva IV catheter, Becton–Dickinson, Franklin Lakes, NJ) and collected in Vacutainers (Becton–Dickinson, Franklin Lakes, NJ), containing EDTA. Initial placement of the catheter was followed by a resting period of 30 min to ensure recovery from potential stress response to catheter placement or traveling to the laboratory. Because previous research has found that IL-6 peaks 120 minutes post-stressor (von Kanel, Kudielka et al. 2006, Breines, Thoma et al. 2014, Rohleder 2014), blood was drawn at baseline, 30, and 120 min following the TSST on both study days.
3.2.1 Stress Induction Paradigm
Acute psychosocial stress was induced using the Trier Social Stress Test (TSST, (Kirschbaum, Pirke et al. 1993)), a widely used standardized laboratory stress paradigm. The TSST used in the present study consisted of a three-minute preparation period, a five-minute public speech, and a five-minute mental arithmetic task in front of an audience of two judges wearing lab coats and maintaining a neutral evaluative facial expression. The public speech involved describing how one’s personality makes one qualified for a dream job and the mental arithmetic ask involved counting backwards from 2043 by deduction of 17 on the first study day and from 2011 by 13 on the second study day. Participants were informed that the judges were trained in analyzing verbal and non-verbal behavior and that their performance would be videotaped. The TSST has demonstrated reliability and validity and has been shown to produce strong biological responses to stress (Dickerson and Kemeny 2004).
3.3 Measures
3.3.1 Self-reports of psychological health
Depressive symptoms were assessed using the 20-item Center for Epidemiologic Studies Depression Scale (CES-D; (Radloff 1977)) by which respondents are asked to indicate how often they have felt or behaved in the stated manner over the past week, including statements such as “I felt depressed.” Ratings are made on a 4-point scale (0 = Rarely or none of the time; 3 = Most or all of the time) and final scores are computed by summing scores on all items after reverse scoring four items. The CES-D has demonstrated reliability and validity (Radloff 1977). In the present study, the CES-D showed good reliability (α= 0.89) and average scores fell below the clinical cut-off of 16 (M = 12.15; SD = 10.02, Range 0–43) (Anderson, Freedland et al. 2001).
Perceived stress over the past month was assessed using the Perceived Stress Scale (PSS, (Cohen, Kamarck et al. 1983)). The PSS is designed to measure how stressful an individual interprets their life to be. Respondents are asked how often they have experienced certain situations and how they have felt about them. Ratings are made on a 5-point scale (0 = Never; 4 = Very Often) and final scores are computed by summing scores on all items after reverse scoring four items. In the present study, the PSS showed good reliability (α = 0.87).
Positive and negative affect post-TSST was assessed using the Positive and Negative Affect Schedule (PANAS, (Watson, Clark et al. 1988)). Respondents are asked how strongly they feel positive (interested, excited, strong, enthusiastic, proud alert, inspired, determined, attentive and active) and negative (distressed, upset, guilty, scared hostile, irritable, ashamed, nervous, jittery and afraid) emotions. Ratings are made on a 5-point scale (1 = very slightly or not at all; 5 = extremely) and greater scores on each subscale indicate greater positive or negative affect. In the present study, the PANAS showed good reliability (positive affect Day 1: α = 0.93; negative affect Day 1: α = 0.90; positive affect Day 2: α = 0.93; negative affect Day 2: α = 0.87).
3.3.2 Measures of adiposity
Weight and body fat measurements were taken using a Seca Supra Plus 720 column scale (Seca, Hamburg, Germany), via a bioelectrical impedance analysis. Briefly, a current is passed through the participant and resistance is measured. The scale uses this feedback to calculate body fat and body water percentage (Foster and Lukaski 1996). Participants were asked to stand barefoot on the scale and two measurements were taken per individual using a normal body type and athletic body type measurement mode. Both measurements were averaged to obtain a measure that was independent of subjective judgment of participants’ body types. Height, waist and hip circumference measurements were taken with a tape measure by the study nurse at the end of the first session. Waist measurements were taken midway between the ileac crest and the lowest rib. Hip measurements were taken at the maximum width of the buttocks or the gluteofemoral fold. BMI was expressed in kg/m2. WHR was calculated as waist circumference divided by hip circumference. Using established WHO guidelines, we split our sample into lean and overweight participants based upon a BMI cutoff of 25 kg/m2 (WHO 2013). This cut-off was used for graphing purposes only. The median BMI was 25. Characteristics of participants included in analyses are presented in Table 1.
Table 1.
Characteristics of Participants
| BMI (kg/m2) | 24.9, SD=3.5 |
| Body fat % | 25.1, SD=6.7 |
| WHR | 0.85, SD=0.08 |
| Waist (cm) | 83.1, SD=11.3 |
| Age (yrs) | 37, SD=17.8 |
| Sex | 32M, 36F |
3.3.3 Measurement of HPA axis and IL-6 stress responses
To assess HPA axis responses to repeated acute stress, we measured salivary free cortisol. Saliva samples were collected at the time points indicated above, salivettes were centrifuged, and saliva was stored at −20 °C until processing. Cortisol was measured using a competitive chemiluminescence immunoassay (CLIA; IBL-International, Toronto, ON, Canada). To assess systemic inflammation at baseline and in response to acute stress, we measured plasma interleukin-6 (IL-6) at baseline (pre-TSST), as well as 30 and 120 min post-TSST on both study days. Blood samples were centrifuged immediately and plasma was aliquoted and stored at −80 °C until batch processing. IL-6 concentrations were determined using a commercial high-sensitivity ELISA (Quantikine HS; R&D Systems, Minneapolis, MN, USA), with a lower limit of detection for IL-6 of 0.09 pg/ml. Inter- and intra assay coefficients of all assays were below 10%.
3.4 Statistical Analyses
All statistical analyses were performed using SPSS 21 for Mac OS X software packages (IBM, Chicago, IL, USA). Kolmogorov–Smirnov tests were computed prior to statistical analyses to test for normal distribution as well as homogeneity of variance of all dependent variables. To test for stress-induced changes in cortisol and IL-6, we used analysis of variance (ANOVA) and analysis of covariance (ANCOVA) for repeated measures, with the within-subject factors “day” (day 1 vs. day 2) and “time” (three time points for IL-6, and six time points for cortisol). We controlled for age and sex where appropriate, and we added the between-subjects factor overweight vs. lean based on a BMI cut-off of 25kg/m2 (see above) to test for differences in stress responses between overweight and lean participants. In all ANOVAs, Greenhouse–Geisser corrections were applied if the sphericity assumption was violated (Vasey and Thayer 1987, Greenhouse and Junker 1992). For an estimation of cortisol stress reactivity to each of the TSSTs, we computed area under the curve with respect to increase (AUCi) indices (Pruessner, Kirschbaum et al. 2003). To estimate IL-6 stress reactivity, we computed delta scores by subtracting pre-stress IL-6 concentrations from IL-6 concentrations two hours post-TSST. To further examine the associations of adiposity measures with cortisol and IL-6 stress responses, we performed linear regression analyses, controlling for age and sex in the first step, and entering each measure of adiposity as a predictor in the second step. All reported results were considered to be significant at the p ≤ 0.05 level and were considered a trend at the p ≤ 0.1 level. Unless otherwise indicated, all reported values shown are untransformed means ± standard deviations (SD).
4 Results
4.1 Preliminary Analyses
Women had higher body fat (M=27.3%, SD=5.4) than men (M=22.8%, SD=7.3; t(64)=−2.9, p=0.005), lower WHR (M=0.80, SD=0.06) than men (M=0.90, SD=0.07) t(66)=5.9, p<0.001, as well as a lower waist circumference (M=77.8cm, SD=8.6cm) than men (M=90cm, SD=10.8cm) t(66)=5.0, p<0.001). There was no significant difference in BMI between men and women. There were no correlations between BMI, body fat, WHR and waist circumference with age, CES-D, and PSS except for waist with age (r=0.35, p=0.004), and body fat with PSS (r=0.35, p=0.005). There was no correlation between CES-D or PSS scores with baseline or stress responses of cortisol or IL-6 on either of the study days. Higher waist circumference was related with lower negative affect on day one (r=−0.26, p=0.033), but not on day two (r=−0.21, p=0.10).
4.2 Adiposity and HPA axis
4.2.1 Cortisol Stress Response
We first examined whether repeated TSST exposure induced increases in salivary cortisol using repeated measures ANOVA. There was a significant time effect (F2.4,118=38.0, p<0.001) indicating cortisol responses to stress, and a significant day by time interaction (F3.0,144.7=8.2, p=<0.001) indicating habituation of cortisol responses on the second day. Further, we found a significant day by time by sex interaction indicating that men and women showed differences in HPA axis response and habituation, such that men showed stronger cortisol responses to TSST1 and greater habituation to TSST2 (F3.0,144.6=2.9, p=0.038). Additionally, there was a negative relationship between age and cortisol increases (AUCi) to TSST1 (beta = −0.31, p=0.022; R2 = 0.01).
4.2.2 Adiposity and Baseline Cortisol
As shown in table 3, WHR and waist circumference were significantly or marginally significantly related to baseline cortisol on day 1, while BMI and body fat percentage were not related to baseline cortisol on day 1, when controlling for age and sex. BMI, body fat percentage and waist circumference were significantly, or marginally significantly, related with higher baseline cortisol concentrations on day 2, controlling for age and sex.
Table 3.
Relationships between measures of adiposity and cortisol baseline and responses
| Cortisol baseline day 1 |
Cortisol baseline day 2 |
Cortisol response day 1 |
Cortisol response day 2 |
|
|---|---|---|---|---|
| Body mass index (BMI) | beta=0.15; p=0.27; R2 = 0.09 | beta=0.27; p=0.043; R2 = 0.17 | beta=0.16; p=0.22; R2 = 0.23 | beta=0.34; p=0.014; R2 = 0.17 |
| Body fat % | beta=0.19; p=0.18; R2 = 0.09 | beta=0.26; p=0.064; R2 = 0.12 | beta=0.12; p=0.37; R2 = 0.22 | beta=0.29; p=0.045; R2 = 0.16 |
| Waist circumference | beta=0.29; p=0.09; R2 = 0.12 | beta=0.33; p=0.047; R2 = 0.17 | beta=0.33; p=0.04; R2 = 0.27 | beta=0.39; p=0.02; R2 = 0.17 |
| Waist-to-hip ratio (WHR) | beta=−0.34; p=0.038; R2 = 0.14 | beta=0.15; p=0.93; R2 = 0.10 | beta=0.22; p=0.17; R2 = 0.22 | beta=−0.20; p=0.22; R2 = 0.11 |
All regression analyses controlled for age and sex in the first step.
4.2.3 Adiposity and Cortisol Responses to Repeated Stress
As shown in table 3, BMI, body fat, and WHR were not significant predictors of cortisol response on day 1, while waist circumference was, when controlling for age and sex. BMI, body fat percentage and waist circumference were significant predictors of cortisol increase on day 2, while WHR was not, when controlling for age and sex.
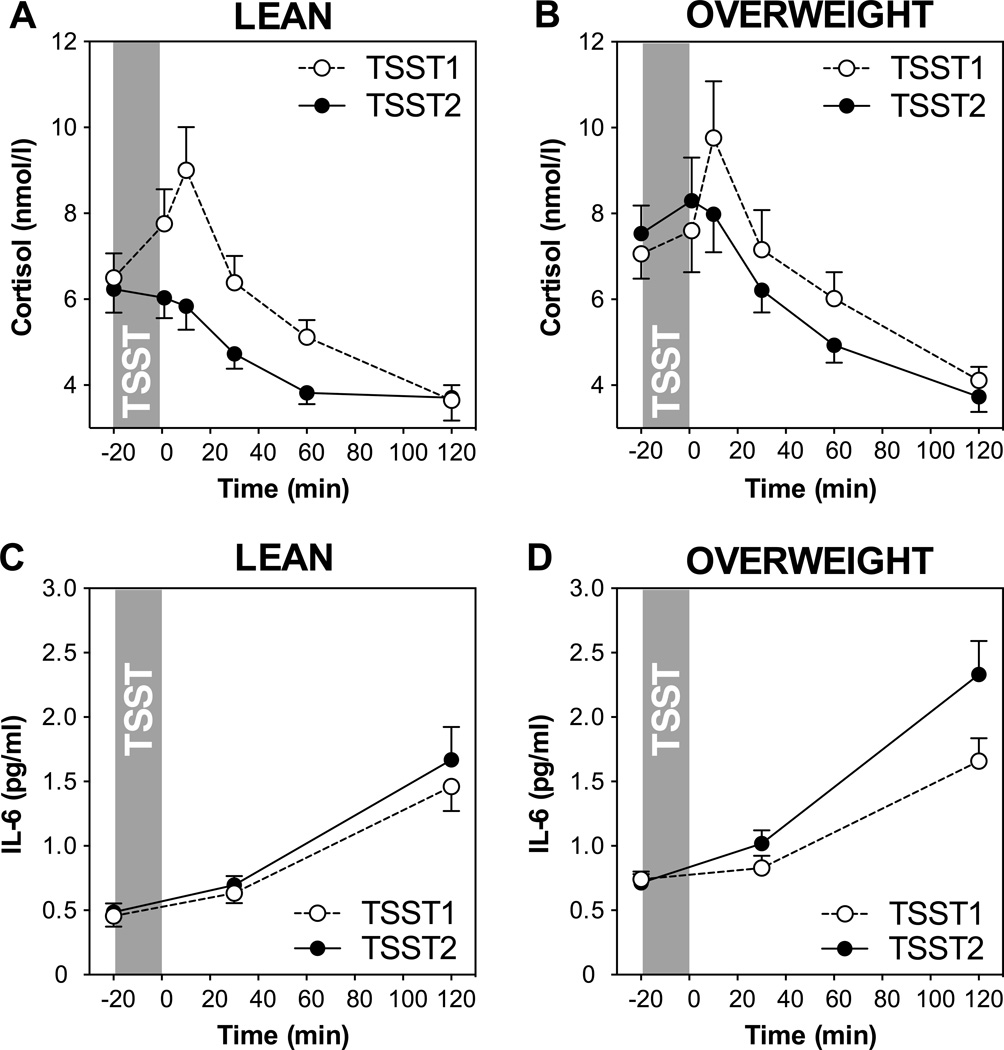
In order to better understand and visualize the relationship of BMI with cortisol responses, we performed a repeated measures ANOVA using BMI-based obesity grouping as a factor after splitting the sample into lean and overweight groups at BMI = 25 kg/m2. As shown in Figures 2A and B, both, lean and overweight participants showed a strong response to the initial TSST and habituation to repeated stress. However, a marginally significant group by day by time interaction indicates less efficient habituation in overweight participants (F3.0,151.9=2.5, p=0.061).
Figure 2.
HPA axis responses to repeated stress in participants below (A: lean, n=19) and above (B: overweight, n=24) the BMI cut-off of 25 kg/m2. Graphs show means and standard errors of the mean (SEM) of salivary cortisol concentrations at baseline as well as 1, 10, 30, 60, and 120 min post-TSST; Inflammatory responses to repeated stress in participants below (C: lean, n=33) and above (D: overweight, n=35) the BMI cut-off. Graphs show means and SEM of plasma IL-6 concentrations at baseline as well as 30 and 120 min post-TSST.
4.3 Adiposity and Plasma Interleukin-6
4.3.1 Interleukin-6 Stress Response
Repeated measures ANOVA of plasma IL-6 on both study days with the factors sex and age group revealed a significant time effect (F1.1,146.2,=90.8, p<0.001), indicating an overall response of IL-6 on both days. We further found a day by time interaction (F1.1,67.6=5.2, p=0.022), indicating differential responses on the two study days. Overall, the IL-6 increase on Day 2 was higher than the increase on Day 1, showing sensitization of IL-6 stress responses (t(63)=−2.54, p=0.012). There were no age or sex group differences in IL-6 responses (all F<1.1; p>0.30). There was no correlation between age and IL-6 baseline or responses (all p’s > 0.46).
4.3.2 Adiposity and Baseline Interleukin-6
As shown in table 4, BMI body fat percentage and waist circumference were significantly, or marginally significantly, related with higher baseline IL-6 concentrations on both study days, with or without controlling for age and sex. WHR was only related with day 1 baseline IL-6.
Table 4.
Relationships between measures of adiposity and IL-6 baseline and responses
| IL-6 baseline day 1 |
IL-6 baseline day 2 |
IL-6 response day 1 |
IL-6 response day 2 |
|
|---|---|---|---|---|
| Body mass index (BMI) | beta=0.41; p=0.001; R2 = 0.16 | beta=0.34; p=0.011; R2 = 0.12 | beta=0.09; p=0.50; R2 = 0.02 | beta=0.27; p=0.044; R2 = 0.073 |
| Body fat % | beta=0.39; p=0.004; R2 = 0.13 | beta=0.40; p=0.003; R2 = 0.16 | beta=0.17; p=0.21; R2 = 0.03 | beta=0.37; p=0.006; R2 = 0.13 |
| Waist circumference | beta=0.39; p=0.017; R2 = 0.09 | beta=0.33; p=0.057; R2 = 0.08 | beta=0.07; p=0.69; R2 = 0.01 | beta=0.17; p=0.34; R2 = 0.02 |
| Waist-to-hip ratio (WHR) | beta=0.42; p=0.01; R2 = 0.10 | beta=0.19; p=0.28; R2 = 0.04 | beta=0.10; p=0.55; R2 = 0.02 | beta=0.05; p=0.77; R2 = 0.01 |
All regression analyses controlled for age and sex in the first step; Neither age nor sex were significant predictors of baseline IL-6 concentrations except for sex on day 2 when including body fat in the model (all other p > 0.08); All regressions were significant also without controlling for age and sex.
All regression analyses controlled for age and sex in the first step; Neither age nor sex were significant predictors of IL-6 increases (all p > 0.28); All regressions were significant also without controlling for age and sex.
4.3.3 Adiposity and Interleukin-6 Stress Response
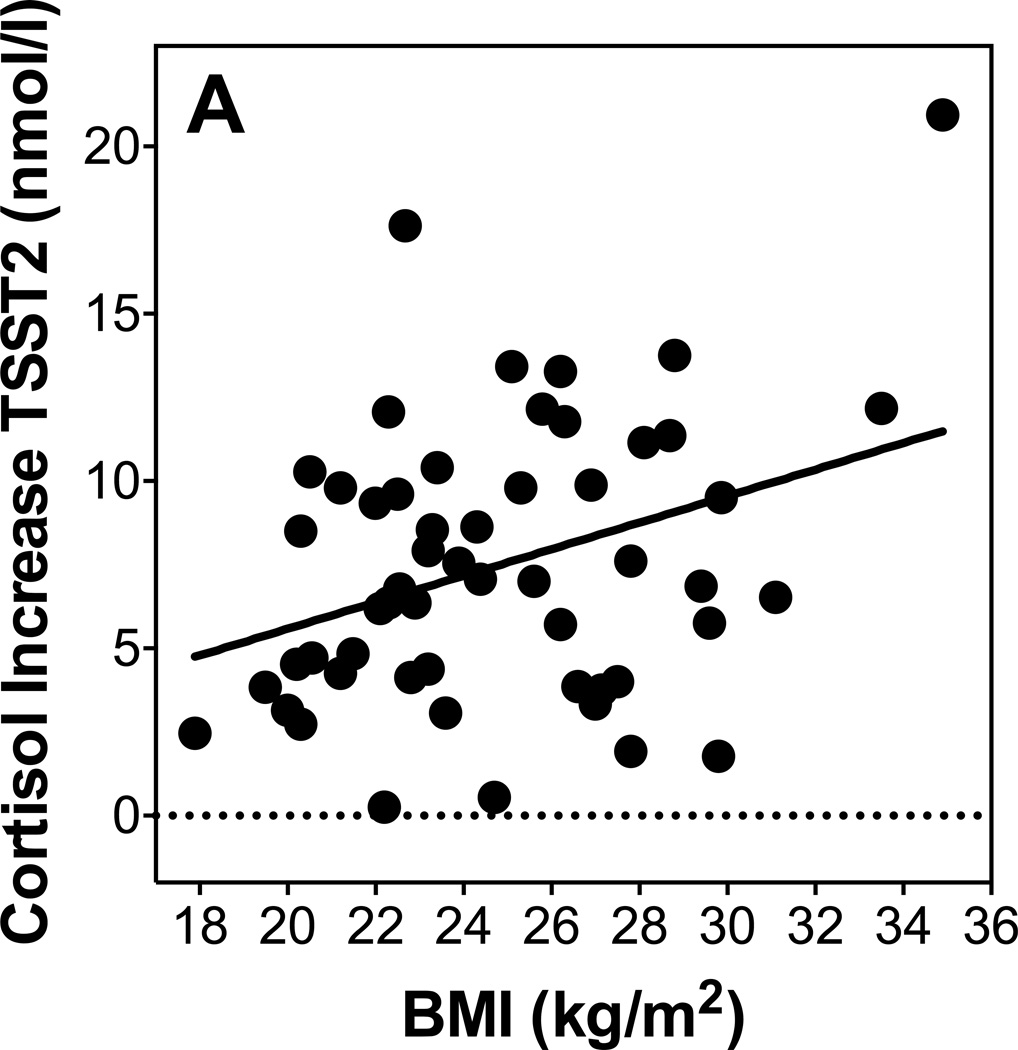
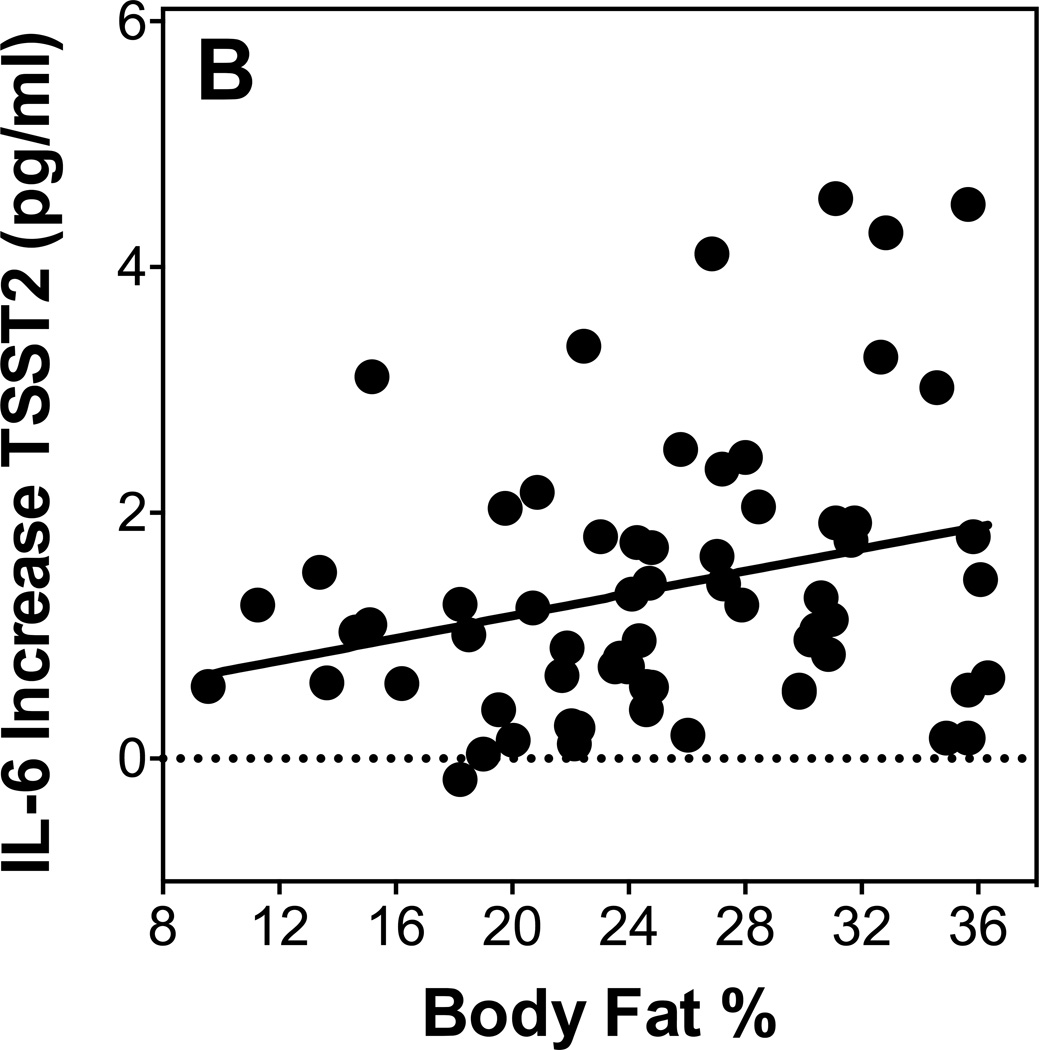
As shown in table 4, none of the adiposity measures were predictors of IL-6 stress responses on day 1, with or without controlling for age and sex. However, IL-6 responses on Day 2 were positively related with BMI and with body fat percentage controlling for age and sex (see Figure 1B). Waist circumference and WHR were not significant predictors of IL-6 response on Day 2 with or without controlling for age and sex.
Figure 1.
Scatterplots showing the relationship of BMI with Cortisol response to TSST2 (A) and IL-6 response to TSST2 (B).
Since depression has been found to be associated with IL-6 responses to stress, and with adiposity (Roberts, Kaplan et al. 2000, Fagundes, Glaser et al. 2013), we additionally controlled for CES-D to ensure that increased depression in overweight individuals was not driving the relationship between adiposity and sensitized IL-6 responses on day 2. BMI remained a marginally significant predictor of IL-6 increase on day 2 (beta=0.25; p=0.07; R2 = 0.08) when controlling for age, sex, and CES-D (CES-D: beta=0.09). Body fat remained a significant predictor of IL-6 increase on day 2 (beta=0.36; p=0.009; R2 = 0.14) when controlling for age, sex, and CES-D (CES-D; beta=0.08). Waist circumference and WHR remain unrelated with IL-6 responses when controlling for CES-D (lowest p=0.44).
Controlling for PSS, our main findings were also remained significant or marginally significant. BMI remained a marginally significant predictor of IL-6 increase on day 2 (beta=0.25; p=0.07; R2 = 0.08) when controlling for age, sex and PSS (PSS beta=0.10). Body fat remained a significant predictor of IL-6 increase on day 2 (beta=0.36; p=0.011; R2 = 0.13) when controlling for age, sex and PSS (PSS beta=0.033). Waist circumference and WHR remain unrelated with IL-6 responses when controlling for PSS (lowest p=0.44).
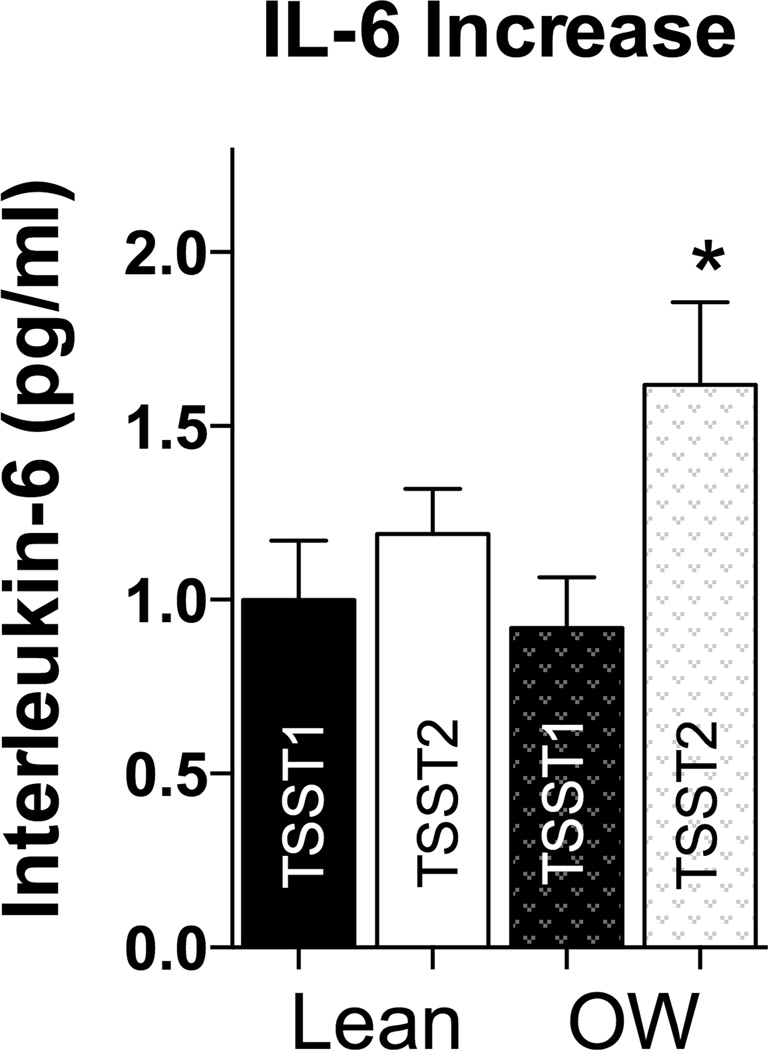
In order to better understand and visualize the relationship of BMI with IL-6 responses to repeated stress, we again used the split into lean and overweight participants using a BMI cutoff of 25 kg/m2. As shown in Figures 2C and D, overweight individuals showed similar IL-6 increases on day 1, but higher increases than normal weight individuals on day 2. While repeated measures ANOVA did not reveal any significant day, time, or day by time effects with our grouping factor (p > 0.20), paired samples t-tests comparing IL-6 increases from baseline to peak revealed that IL-6 responses were significantly larger on Day 2 compared to Day 1 in the overweight group (t(32)=−2.54, p=0.016), but not the lean group (t(30)=−0.95, p=0.35, see Figure 3), indicating sensitization of IL-6 stress responses.
Figure 3.
No difference in IL-6 response to TSST1 and TSST2 in lean individuals, but overweight (OW) individuals had a significantly greater increase in IL-6 in response to TSST2 than TSST1 in overweight individuals.
4.4 Relationship between HPA axis and IL-6 responses to repeated stress
To test whether greater cortisol responses were associated with lower IL-6 increases, we used linear regression. The relationship between cortisol responses and IL-6 responses were not significant on either study day (p>0.30).
5 Discussion
In this study, we aimed to investigate whether measures of adiposity would be related with HPA axis and IL-6 responses, as well as habituation or sensitization of these systems, in response to repeated acute psychosocial stress. Results confirmed previous findings of stress response of the HPA axis and habituation, as well as stress responses and non-habituation of IL-6 responses to repeated stress in the total sample (Schommer, Hellhammer et al. 2003, von Kanel, Kudielka et al. 2006). Measures of adiposity were related with higher IL-6 baselines on both study days, and with cortisol baseline on the day of repeated stress exposure. With regard to our main hypotheses, we found that higher BMI and body fat percentage were related with higher cortisol stress responses to repeated stress, indicating less efficient habituation. Similarly, IL-6 responses to repeated stress were positively related with BMI and body fat, indicating sensitization of stress responses, in line with our hypotheses that higher measures of adiposity would be related with altered endocrine and IL-6 stress responses.
Our findings of less efficient HPA axis habituation in overweight individuals, as well as the higher day 2 baseline cortisol values, suggest maladaptive alterations of HPA axis stress responses in overweight individuals. Elevated basal cortisol secretion has been linked with excess central fat, in some (Mayo-Smith, Hayes et al. 1989, Trayhurn 2005), but not all studies (Epel et al., 2000). We found here that individuals with higher WHR had increased baseline cortisol only on day 1. However, the fact that in our study, we found relationships of baseline cortisol with BMI, body fat and waist circumference only on the second day of stress testing supports the alternative interpretation that overweight individuals might come into repeated stress testing with higher anticipatory stress. With regard to stress responses, we found that waist circumference was positively related to cortisol stress responses on both days, while BMI and body fat were predictors of cortisol responses to repeated exposures only. Earlier studies have revealed mixed findings, with some studies reporting positive associations of measures of central (Marin, Darin et al. 1992, Moyer, Rodin et al. 1994) and total adiposity (Benson, Arck et al. 2009) with cortisol stress responses, and others reporting lower cortisol responses to onetime stress exposure in individuals with higher body fat (Jones et al., 2012). Our findings of a correlation of initial cortisol stress responses with waist circumference, but not with WHR, BMI, and body fat percentage, is only partially in line with this literature. These differences might be explained by different gender and age distribution, as well as different ranges of adiposity between these studies. Further, there seems to be a complex relationship between different measures of adiposity. Jones et al. (2012) used a MR imaging based method of body fat assessment and found lower HPA axis responses in relation to both BMI and visceral fat volume, while Epel et al. (2000) found higher HPA axis responses related with higher central adiposity, but no significant differences between high and low BMI groups. Epel et al.’s study is also the only other study employing repeated stress exposure and our findings of less efficient HPA axis habituation in individuals with higher BMI and body fat percentage is in line with their results, but extends it to both genders and a larger age range (Epel, McEwen et al. 2000). Since our study was cross-sectional, we cannot determine whether obesity caused the altered HPA axis activity and reactivity found here, or whether altered HPA axis reactivity affects eating patterns and thereby might cause obesity. Studies showing that individuals with higher cortisol responses to stress consumed more snack foods than individuals with lower cortisol responses might support this alternative pathway or a bi-directional relationship between adiposity and HPA axis stress responses (Macht and Simons 2000, Newman, O'Connor et al. 2007).
Our findings of relationships of baseline IL-6 levels with measures of adiposity are in agreement with the well-documented relationship of adiposity with chronic low-grade inflammation (Rexrode, Pradhan et al. 2003, Panagiotakos, Pitsavos et al. 2005, Himmerich, Fulda et al. 2006, Thorand, Baumert et al. 2006, Brydon 2011). Only one prior study found that waist circumference was related to baseline IL-6, but not with IL-6 responses to a single laboratory stress task (Brydon 2011). Consistent with this, we found in our study that IL-6 stress responses were not related with waist circumference or WHR. Above and beyond these earlier results, however, we found here that when exposed to the same stressor repeatedly, individuals with higher BMI and body fat percentage showed significantly higher IL-6 responses to this repeated stressor, indicating sensitization of acute stress responses.
While the current findings clearly document that individuals with higher body fat percentage have higher IL-6 responses when exposed to the same stressor repeatedly, it remains an open question where the additional stress-induced plasma IL-6 protein is originating, and how this release is stimulated. Adipose tissue is the likely source, as adipocytes secrete IL-6 and other pro-inflammatory cytokines (Trayhurn and Wood 2004). Furthermore, adipose-tissue infiltrating macrophages secrete inflammatory cytokines, and adipose expression of cytokines is correlated with peripheral levels of these plasma proteins (Weisberg, McCann et al. 2003, Trayhurn and Wood 2004, Lasselin, Magne et al. 2014). Increased leptin levels in response to stress may drive synthesis of cytokines by macrophages, as women with larger waists showed higher leptin responses, and higher basal leptin was associated with greater IL-6 responses (Brydon 2011). Leptin receptors are present on macrophages, and binding stimulates macrophage production of IL-6 (Gabay, Dreyer et al. 2001, La Cava and Matarese 2004). It is therefore conceivable that larger leptin responses in overweight individuals result in greater IL-6 responses to stress. Analysis of stress-induced leptin levels in a group with an established relationship between adiposity and plasma IL-6 response might shed light on whether the greater stress-induced IL-6 increase in overweight individuals is due to an increased leptin response to stress.
Higher IL-6 responses could theoretically be the result of lower HPA axis responses, due to the well-described inhibitory effects of glucocorticoids on the inflammatory cascade in general (Munck, Guyre et al. 1984, Sapolsky, Romero et al. 2000), and because intracellular inflammatory responses to stress have been found to be inversely related with cortisol responses in particular (Wolf, Rohleder et al. 2009). However, our data do not support this possibility, because we find higher, not lower cortisol responses to repeated stress in individuals with higher measures of adiposity, and we also do not find cortisol and IL-6 responses to be related on either of the two days of stress exposure.
Chronic low-grade inflammation, indicated by increased concentrations of IL-6 and C-reactive protein, contributes to conditions responsible for some of the most common causes of morbidity and mortality, including Type 2 Diabetes, atherosclerosis, heart disease and cancer (Danesh 1999, Roberts, Dive et al. 2010, Kotas, Gorecki et al. 2013), which provides an important link in obesity-related chronic diseases (Rexrode, Pradhan et al. 2003, Panagiotakos, Pitsavos et al. 2005, Himmerich, Fulda et al. 2006, Thorand, Baumert et al. 2006, Brydon 2011). The finding that overweight individuals also display sensitization of stress-induced increases in IL-6 is especially concerning in conjunction with their well-documented systemic low-grade inflammation. We see here that a dysregulated IL-6 response to stress compounds the pre-existing condition of increased peripheral inflammation in obesity. Our work thereby underscores the importance of weight maintenance in regards to the ability to mount adaptive stress responses, and reducing risk for stress- and inflammation-driven co-morbidities of obesity.
The current findings have to be interpreted in light of some limitations. First of all, the findings were based on the correlational relationships between measures of adiposity and endocrine and IL-6 stress responses. To draw causal conclusions, a prospective investigation or time-dependent experimental manipulation would be necessary, for example employing a weight-loss intervention. However, given the current state of our knowledge, this cross-sectional design provides a significant improvement and groundwork for future studies, especially findings of acute stress responses. Additionally, it may be possible that the stress effects of session one have not completely recovered before session two. This does not seem to be an issue for plasma IL-6, but may in fact be occurring for cortisol in the overweight group as their baseline cortisol on session two was elevated. Also, our sample size was relatively small, and a larger sample size may have allowed us to detect more subtle relationships. Finally, as our screening criteria required that individuals be free of potentially confounding health conditions, we had a limited number of obese individuals included in our analyses. Having a larger sample of obese participants may further increase the generalizability of these findings to obese individuals and may have allowed us to detect larger group differences and greater effects of adiposity.
Taken together we show here that measures of adiposity are related with higher sensitization of IL-6 responses to acute stress, and with altered HPA axis responses to repeated stress, characterized by higher baseline cortisol before repeated stress exposure, and most importantly, by less efficient habituation. Future studies should investigate whether overweight individuals show decreased GC sensitivity on the second day, which could explain their sensitized IL-6 response without corresponding decreased cortisol response and in fact decreased glucocorticoid sensitivity has been reported in individuals with higher BMI (Wirtz, Ehlert et al. 2008). Additionally, examining leptin response in conjunction with plasma IL-6 response to stress in a population with a detectable influence of adiposity on IL-6 response would begin to help address the question of whether increased leptin response drives increased immune response in overweight and obese individuals.
Table 2.
Characteristics of Participants included in IL-6 analysis
| Lean | Overweight | ||
|---|---|---|---|
| BMI (kg/m2)*** | 22.1, SD=1.8 | 27.7, SD=2.4 | t(66)=−11.1, p<0.001 |
| Body fat %*** | 21.8, SD=5.9 | 28.5, SD=5.7 | t(64)=−4.7, p<0.001 |
| WHR* | 0.82, SD=0.07 | 0.87, SD=0.09 | t(66)=−2.4, p=0.021 |
| Waist (cm)*** | 76.2, SD=7.5 | 89.8, SD=10.5 | t (66)=−6.3, p<0.001 |
| Age (yrs) | 36.1, SD=18.2 | 37.6, SD=17.6 | t(66)=−0.4, ns |
| Sex | 13M, 20F | 19M, 16F | Χ2=1.5, p=0.22 |
Note:
p<0.05,
p<0.01,
p<0.001
Note: There were no differences in these characteristics between the larger sample used for IL-6 analysis and the smaller sample for cortisol analysis
Research Highlights.
Measures of adiposity are related with higher sensitization of IL-6 responses to repeated stress.
Measures of adiposity are further related with higher cortisol baseline before repeated stress exposure, and less efficient habituation of cortisol stress responses.
Acknowledgements
This research was supported by the American Federation of Aging Research (NR), and by training grants from the National Institute of Health (T32 MH 019929: CM, T32-084907 DG). MVT acknowledges funding from the Swiss National Science Foundation (SNF). SH acknowledges funding, R01 HL90975 from the National Institues of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare that there are no conflicts of interest.
References
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Benson S, Arck PC, Tan S, Mann K, Hahn S, Janssen OE, Schedlowski M, Elsenbruch S. Effects of obesity on neuroendocrine, cardiovascular, and immune cell responses to acute psychosocial stress in premenopausal women. Psychoneuroendocrinology. 2009;34(2):181–189. doi: 10.1016/j.psyneuen.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Visceral obesity: a "civilization syndrome". Obes Res. 1993;1(3):206–222. doi: 10.1002/j.1550-8528.1993.tb00614.x. [DOI] [PubMed] [Google Scholar]
- Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52(1):1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Breines JG, Thoma MV, Gianferante D, Hanlin L, Chen X, Rohleder N. Self-compassion as a predictor of interleukin-6 response to acute psychosocial stress. Brain Behav Immun. 2014;37:109–114. doi: 10.1016/j.bbi.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L. Adiposity, leptin and stress reactivity in humans. Biol Psychol. 2011;86(2):114–120. doi: 10.1016/j.biopsycho.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Danesh J. Smoldering arteries? Low-grade inflammation and coronary heart disease. JAMA. 1999;282(22):2169–2171. doi: 10.1001/jama.282.22.2169. [DOI] [PubMed] [Google Scholar]
- Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62(5):623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Hwang BS, Malarkey WB, Kiecolt-Glaser JK. Depressive symptoms enhance stress-induced inflammatory responses. Brain Behav Immun. 2013;31:172–176. doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KR, Lukaski HC. Whole-body impedance--what does it measure? Am J Clin Nutr. 1996;64(3 Suppl):388S–396S. doi: 10.1093/ajcn/64.3.388S. [DOI] [PubMed] [Google Scholar]
- Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier CA. Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab. 2001;86(2):783–791. doi: 10.1210/jcem.86.2.7245. [DOI] [PubMed] [Google Scholar]
- Greenhouse JB, Junker BW. Exploratory statistical methods, with applications to psychiatric research. Psychoneuroendocrinology. 1992;17(5):423–441. doi: 10.1016/0306-4530(92)90001-n. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Obesity, metabolic syndrome, and coronary atherosclerosis. Circulation. 2002;105(23):2696–2698. doi: 10.1161/01.cir.0000020650.86137.84. [DOI] [PubMed] [Google Scholar]
- Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, Gedrich K, Pollmacher T. TNF-alpha, soluble TNF receptor and interleukin-6 plasma levels in the general population. Eur Cytokine Netw. 2006;17(3):196–201. [PubMed] [Google Scholar]
- Jones A, McMillan MR, Jones RW, Kowalik GT, Steeden JA, Deanfield JE, Pruessner JC, Taylor AM, Muthurangu V. Adiposity is Associated With Blunted Cardiovascular, Neuroendocrine and Cognitive Responses to Acute Mental Stress. PLoS One. 2012;7:e39143. doi: 10.1371/journal.pone.0039143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74(4):761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- Kotas ME, Gorecki MC, Gillum MP. Sirtuin-1 is a nutrient-dependent modulator of inflammation. Adipocyte. 2013;2(2):113–118. doi: 10.4161/adip.23437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4(5):371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984;289(6454):1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasselin J, Magne E, Beau C, Ledaguenel P, Dexpert S, Aubert A, Laye S, Capuron L. Adipose inflammation in obesity: relationship with circulating levels of inflammatory markers and association with surgery-induced weight loss. J Clin Endocrinol Metab. 2014;99(1):E53–E61. doi: 10.1210/jc.2013-2673. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- Macht M, Simons G. Emotions and eating in everyday life. Appetite. 2000;35(1):65–71. doi: 10.1006/appe.2000.0325. [DOI] [PubMed] [Google Scholar]
- Marin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41(8):882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- Mayo-Smith W, Hayes CW, Biller BM, Klibanski A, Rosenthal H, Rosenthal DI. Body fat distribution measured with CT: correlations in healthy subjects, patients with anorexia nervosa, and patients with Cushing syndrome. Radiology. 1989;170(2):515–518. doi: 10.1148/radiology.170.2.2911678. [DOI] [PubMed] [Google Scholar]
- Moyer AE, Rodin J, Grilo CM, Cummings N, Larson LM, Rebuffe-Scrive M. Stress-induced cortisol response and fat distribution in women. Obes Res. 1994;2(3):255–262. doi: 10.1002/j.1550-8528.1994.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Newman E, O'Connor DB, Conner M. Daily hassles and eating behaviour: the role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32(2):125–132. doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183(2):308–315. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann Epidemiol. 2003;13(10):674–682. doi: 10.1016/s1047-2797(03)00053-x. [DOI] [PubMed] [Google Scholar]
- Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Are the obese at greater risk for depression? Am J Epidemiol. 2000;152(2):163–170. doi: 10.1093/aje/152.2.163. [DOI] [PubMed] [Google Scholar]
- Rohleder N. Stimulation of Systemic Low-Grade Inflammation by Psychosocial Stress. Psychosom Med. 2014 doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenalmedullary system to repeated psychosocial stress. Psychosom Med. 2003;65(3):450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Thorand B, Baumert J, Doring A, Herder C, Kolb H, Rathmann W, Giani G, Koenig W, Group K. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis. 2006;184(1):216–224. doi: 10.1016/j.atherosclerosis.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184(4):285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology. 1987;24(4):479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav Immun. 2006;20(1):40–48. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Geneva: World Health Organization; 2013. Obesity and Overweight. Fact Sheet No. 311. [Google Scholar]
- Wirtz PH, Ehlert U, Emini L, Suter T. Higher body mass index (BMI) is associated with reduced glucocorticoid inhibition of inflammatory cytokine production following acute psychosocial stress in men. Psychoneuroendocrinology. 2008;33(8):1102–1110. doi: 10.1016/j.psyneuen.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Rohleder N, Bierhaus A, Nawroth PP, Kirschbaum C. Determinants of the NF-kappaB response to acute psychosocial stress in humans. Brain Behav Immun. 2009;23(6):742–749. doi: 10.1016/j.bbi.2008.09.009. [DOI] [PubMed] [Google Scholar]