Abstract
Present study was carried out to evaluate the potential of Lactobacillus acidophilus (L. acidophilus) for development of wheat based probiotic beverage and to optimize the proportion of different ingredients viz. sprouted wheat flour, sprouted wheat bran, oat and stabilizer using response surface methodology. Acidity, pH and probiotic count of samples prepared with L. acidophilus NCDC-14 was higher than that of L. acidophilus NCDC-16 culture. Being more compatible, L. acidophilus NCDC-14 was selected for this study. Acidity (in terms of lactic acid), pH and probiotic count of the different samples ranged from 0.21 to 0.45 %, 4.0 to 4.9, and 8.30 to 10.95 log10 cfu mL−1, respectively. Probiotic count increased with increasing amount of sprouted wheat and oat. Optimized levels for sprouted wheat flour, oat, wheat bran and guar gum were 7.86, 5.42, 1.42 and 0.6 g, respectively per 100 mL of water. Optimized probiotic beverage provided 13.19 % total solids, 1.19 % protein, 0.33 % fat, 0.10 % ash, 0.42 % crude fibre, 1.45 mg iron, calcium 15.74 mg, 11.56 % carbohydrates, 54 kcal calories and 10.43 log10 cfu mL−1 probiotic count. Thus, Lactobacillus acidophilus NCDC-14 can be used for development of potentially probiotic beverage with sprouted wheat and oat.
Keywords: Lactobacillus acidophilus-NCDC14, Sprouted wheat, Probiotic beverage
Introduction
Tremendous changes in lifestyle, eating habits and shifting rural habitations are causing an irreversible change that is leading to manifold multiplication of health problems. Due to huge expenditure on health care each year, consumers’ desire for food products with desired health benefits continues to grow. Consumers are interested in foods that boost the immune system, reduce the risk of disease and enhance health, which consumers self-prescribe for themselves and their families. Nowadays functional foods are gaining public acceptance in many countries. The market surveys showed that there is great scope for value-added as well as health promoting food products (Singh 2007).
A major development in functional foods pertains to foods containing probiotics and prebiotics which enhance health promoting microbial flora in the intestine. There is growing scientific evidence to support the concept that live microorganisms when administered in adequate amounts confer a health benefit on the host by improving its intestinal microbial balance (FAO/WHO 2001; Holzapfel et al. 2001; Fuller 1992). A number of genera of bacteria (and yeast) are used as probiotics, including Lactobacillus, Leuconostoc, Pediococcus, Bifidobacterium, and Enterococcus, but the main species believed to have probiotic characteristics are L. acidophilus, Bifidobacterium spp., and L. casei (Sharma and Mridula 2013). Considering the different intestinal bacterial groups, it is well known that bifidobacteria and lactobacilli can be used as probiotics, i.e. live microbial food ingredients that are beneficial to health. Various efforts have been made in order to increase in the colon the number and/or the activity of the bacterial groups considered beneficial for the host and to decrease those considered as harmful (Matteuzzia et al. 2004). By increasing the amount of prebiotics in the diet, it is possible to increase and maintain healthy bacterial gut flora in the host (Gibson et al. 2003). Ingredients in certain food products may naturally contain prebiotics which help to improve the functional efficacy of probiotics. Foods can also be fortified with prebiotics during manufacturing process to increase probiotic efficacy (Ranadheera et al. 2010). When both prebiotics and probiotics are present in a food then those functional foods are referred to as synbiotic (Pandiyan et al. 2012).
Foods used for dissemination of probiotics are usually fermented foods however, probiotics could also be present in infant formula, fruit drinks, whey drinks and sweet milk. Probiotic LAB (Lactic acid bacteria), especially Lactobacillus and Bifidobacterium, are known to enhance the capacity of host to fight against intestinal infections by stimulating the mucosal immune system (Erickson and Hubbard 2000). Lactobacillus acidophilus strains are widely used as probiotic cultures in dairy products because this species possess therapeutic properties (Pandiyan et al. 2012). Due to the proven health-promoting effects, the lactobacilli have commonly been marketed as probiotics (Shah 2000; Tannock 2004; Bernardeau et al. 2006; Saran et al. 2012). In recent years, cereals have also been investigated regarding their potential use in developing functional foods. Lactic acid fermentation of cereals is a long-established processing method and is being used in Asia and Africa for the production of foods in various forms such as beverages, gruels, and porridge. Cereals contain water-soluble fiber (such as β-glucan and arabinoxylan), oligosaccharides (such as galacto-and fructo oligosaccharides) and resistant starch, and thus have been suggested to fulfill the prebiotic concept (Shah 2001). Whole grains are also sources of many phytochemicals, including phytoestrogens, phenolic compounds, antioxidants, phytic acid and sterols. Lactic acid fermentation usually improves the nutritional value and digestibility of cereals (Charalampopoulos et al. 2002). Lactic acid fermentation of different cereals, such as maize, sorghum, finger millet, has been found effective in reducing the amount of phytic acid, tannins and improve protein digestibility (Chavan et al. 1988; Lorri and Svanberg 1993). In a food product, concentration of approximately 107 probiotic bacterial cells/ml at the time of consumption is considered functional (Gomes and Malcata 1999; Shortt 1999). The Lactobacillus acidophilus strains have been used as probiotic bacteria in various food formulations such as yoghurt, curd, ice cream (Bajad et al. 2006; Yadav et al. 2007; Jain et al. 2008; Pandiyan et al. 2012). Arora et al. (2010) had also developed barley based probiotic food mixture using Lactobacillus acidophilus and recommended a combination of germination and fermentation as a potential process for enhancing the nutritional quality of cereal based food mixes. Freeze dried cultures of Lactobacillus acidophilus NCDC 14, has proven therapeutic benefits (Reddy et al. 2006; Singh et al. 2007).
The objective of this study was to evaluate the potential of L. acidophilus- NCDC14 for development of wheat based probiotic beverage (WPB) and to optimize the proportion of different ingredients viz. sprouted wheat flour, sprouted wheat bran, oat and stabilizer i.e. guar gum with the aim of maximizing probiotic count for preparation of probiotic beverage.
Material & methods
Raw materials
Wheat (cv.PBW550) were cleaned, washed and soaked in water in the ratio of 1:2 (seeds to water) for 8 h at room temperature. After draining the water, the grains were allowed to sprout at controlled temperature (35 °C) and 95 % Relative Humidity. The sprouted wheat was dried at 50 °C in a cabinet tray dryer to 8.86 % moisture level. The rootlets of sprouted and dried wheat were removed by hand scrubbing. Wheat bran was obtained by pearling sprouted wheat for 1 min using grain pearler (Make: CIAE Bhopal, 100–300 kg/h). Oat (Quaker oats) and Guar Gum (SD Fine Chem. Ltd.) were procured from local market. Finally, sprouted wheat, bran and oat were ground and sieved through 85 mesh sieve to obtain a fine formulation (particle size 0.177 mm).
Probiotic cultures
Two probiotic strains namely L. acidophilus NCDC 14 and L. acidophilus NCDC 16 were procured from National Collection of Dairy Cultures, NDRI, Karnal, Haryana, India. The strains were maintained at 4 °C and sub cultured monthly on slants prepared from MRS (de Man Rogosa Sharpe) agar.
Activation of microbial culture and extraction of pellet
Culture was activated in MRS broth by transferring 0.1 mg of freeze dried culture in 10 ml of MRS broth and the tube was incubated at 37 °C for 24 to 48 h. From this 10 ml, 1 ml was taken in 100 mL MRS broth and this culture was reactivated at 37 °C for 24–48 h with several transfers (6 times in 10 mL) of the culture. At last 1 mL from the last 10 mL was taken in 100 mL MRS Broth and incubated at 37 °C for 24–48 h. 1 mL of the activated culture was placed on MRS Agar at 37 °C for 48 h. After 48 h, colonies were picked and gram staining was done for checking the purity of the culture. Rod shaped pink coloured colonies were observed under microscopic and these were picked and their growth was observed in MRS broth at 37 °C for 24–48 h.
Extraction of microbial cell pellets
The activated culture was centrifuged in sterilized centrifuge bottles at 4,500 rpm for 10 min at 4 °C using a bench top refrigerated centrifuge. After centrifugation, supernatant portion of the tube was decanted and microbial pellets were washed in sterilized bottle using sterilized 25–30 ml deionized water/peptone water and centrifuged. The washed pellets were re-centrifuged at 4,500 rpm for 10 min at 4 ºC to remove traces of MRS broth. The cell concentration was tested using pour plate technique and then adjusted to ~ 10 log10CFU per mL by suspending in 0.1 % peptone solution (w/v).
Selection of probiotic bacteria
Sprouted wheat flour mixture (10 g per 100 mL), prepared in sterile water was heated to 90 °C and hold for 5 min. After cooling the flour mixture at room temperature (about 30–35 °C), 1 % (v/v) probiotic culture (probiotic count adjusted to ~ 8 log10CFU per mL) was added and incubated for at 37 °C. Both L. acidophilus NCDC 14 and L. acidophilus NCDC 16 were added separately to these wheat flour slurries. Control samples without any wheat flour (ie. Only containing sterile water and probiotic culture) were also prepared in the similar manner.
Enumeration of probiotic count was done after 4, 5, 6, 7, 8, 9 and 10 h by the pour plate method as described by Shah (2000) using MRS agar. Enumeration of probiotic count was done Sterile peptone solution (0.1 % w/v) was used for making dilutions of samples.
Experimental design
The amount of sprouted wheat flour, oat, sprouted wheat bran and stabilizer (guar gum) were optimized using the Box-Behnken design of response surface methodology (RSM). This design was preferred, as it is made to require only 3 levels, coded as −1, 0, and +1. Box-Behnken designs are available for 3 to 21 factors. They are formed by combining two-level factorial designs with incomplete block designs. This procedure creates designs with desirable statistical properties but, most importantly, with only a fraction of the experiments required for a three-level factorial. The levels of different independent variables and plan of experiment for the present study are given in Table 1. Based upon the statistical analysis of the data, three optimized formulations were selected with desirability level >0.87 for validation purposes (Table 6).
Table 1.
Box-Behnken design with values of independent and dependent variables of WPB
| Experiments | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Response 1 | Response 2 | Response 3 |
|---|---|---|---|---|---|---|---|
| Sprouted wheat flour g/100 ml | Oat g/100 ml | Sprouted wheat bran g/100 ml | Guar gum g/100 ml | pH | Acidity (% in lactic acid) | Probiotic count (log10 cfu mL−1) | |
| 1 | 6 | 2 | 2 | 0.2 | 4.4 | 0.37 | 8.30 |
| 2 | 6 | 6 | 1 | 0.4 | 4.1 | 0.45 | 10.30 |
| 3 | 8 | 4 | 3 | 0.4 | 4.4 | 0.39 | 9.48 |
| 4 | 8 | 4 | 2 | 0.6 | 4.7 | 0.225 | 9.78 |
| 5 | 6 | 2 | 3 | 0.4 | 4.29 | 0.41 | 8.48 |
| 6 | 6 | 4 | 3 | 0.6 | 4.4 | 0.38 | 8.78 |
| 7 | 4 | 4 | 2 | 0.6 | 4.6 | 0.27 | 8.85 |
| 8 | 6 | 4 | 3 | 0.2 | 4.2 | 0.405 | 8.95 |
| 9 | 8 | 6 | 2 | 0.4 | 4 | 0.45 | 10.70 |
| 10 | 4 | 4 | 1 | 0.4 | 4.9 | 0.315 | 9.70 |
| 11 | 6 | 4 | 1 | 0.6 | 4.2 | 0.425 | 9.60 |
| 12 | 6 | 4 | 2 | 0.4 | 4.4 | 0.375 | 9.70 |
| 13 | 8 | 2 | 2 | 0.4 | 4.51 | 0.225 | 8.85 |
| 14 | 6 | 2 | 1 | 0.4 | 4.3 | 0.405 | 8.70 |
| 15 | 4 | 4 | 3 | 0.4 | 4.7 | 0.32 | 9.60 |
| 16 | 6 | 4 | 2 | 0.4 | 4.77 | 0.38 | 8.48 |
| 17 | 8 | 4 | 1 | 0.4 | 4.1 | 0.435 | 10.48 |
| 18 | 6 | 6 | 3 | 0.4 | 4.4 | 0.405 | 10.95 |
| 19 | 6 | 6 | 2 | 0.2 | 4.6 | 0.45 | 10.78 |
| 20 | 4 | 2 | 2 | 0.4 | 4.54 | 0.225 | 8.78 |
| 21 | 6 | 4 | 2 | 0.4 | 4.3 | 0.225 | 8.60 |
| 22 | 6 | 4 | 2 | 0.4 | 4.7 | 0.21 | 9.90 |
| 23 | 6 | 4 | 1 | 0.2 | 4.4 | 0.36 | 10.30 |
| 24 | 6 | 4 | 2 | 0.4 | 4.8 | 0.27 | 8.60 |
| 25 | 4 | 6 | 2 | 0.4 | 4.27 | 0.36 | 10.85 |
| 26 | 6 | 2 | 2 | 0.6 | 4.71 | 0.28 | 8.70 |
| 27 | 4 | 4 | 2 | 0.2 | 4.81 | 0.25 | 8.85 |
| 28 | 6 | 6 | 2 | 0.6 | 4.1 | 0.45 | 10.70 |
| 29 | 8 | 4 | 2 | 0.2 | 4.27 | 0.415 | 8.78 |
WPB wheat based probiotic beverage; Values of responses are means of three replicates
Table 6.
Optimized solutions with predicted and actual experimental values for WPB
| Solution no. | Level of ingredients (g) | pH | Acidity (% lactic acid) | Probiotic count (log10cfu mL−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sprouted wheat flour | Oat | Sprouted wheat bran | Guar gum | Desirability | Predicted | Exp* | Predicted | Exp* | Predicted | Exp* | |
| 1 | 7.86 | 5.42 | 1.42 | 0.6 | 0.875787 | 4.000001 | 4.18 | 0.483895 | 0.46 | 10.40 | 10.43 |
| 2 | 7.86 | 5.4 | 1.41 | 0.6 | 0.87576 | 4.000001 | 4.21 | 0.484363 | 0.44 | 10.39 | 10.26 |
| 3 | 7.86 | 5.46 | 1.45 | 0.6 | 0.875733 | 3.999999 | 4.09 | 0.483191 | 0.47 | 10.41 | 10.44 |
WPB wheat based probiotic beverage; Values of responses are mean of three replicates; *Experimental
Process for preparation of wheat based probiotic beverage
The level of different ingredients as obtained from the experimental design were mixed in sterile water in the laminar air flow in order to avoid contamination and covered tightly using sterile caps. The mixture was heated to 90 °C for 5 min and cooled to room temperature (about 30–35 °C). 1 % (v/v) L. acidophilus NCDC 14 probiotic culture (probiotic count adjusted to ~ 10 log10 cfu per mL) was added to this flour mixture and incubated for at 37 °C for 8 h. All the experiments were conducted in triplicates.
Proximate composition
Moisture, protein (using the factor 6.5 × N), crude fat, ash, crude fibre, calcium and iron in probiotic samples were determined as per standard methods (AOAC 2000). Total carbohydrate value was obtained by difference. Total calories were calculated by multiplying protein, carbohydrates and fat content by 4, 4 and 9, respectively. All the chemicals used for estimation of proximate composition were of AR grade.
Data analysis
The data were analyzed for three replicates using Design Expert 7.0 software for designing and analyzing of response surface data. This software also optimized the independent variables based on conditions.
Results & discussions
Selection of probiotic bacteria
The probiotic count was enumerated for the sprouted wheat slurry prepared using L. acidophilus NCDC 14 and L. acidophilus NCDC 16 probiotic cultures. The probiotic count was higher in the wheat slurry containing L. acidophilus NCDC 14 and the count was fairly good after 8 h of incubation at 37 °C (Fig. 1). Thus, the time period selected for the development of wheat based probiotic beverage was 8 h. The values for pH, acidity and probiotic count of the samples prepared with L. acidophilus NCDC-14 and L. acidophilus NCDC-16 culture were 5.08 and 5.21, 0.0352 % and 0.0285 % and 8.70 log10 cfu mL−1 and 6.78 log10 cfu mL−1, respectively. Thus, form these experiments; the better compatible strain, i.e. L. acidophilus NCDC-14 was selected for further study.
Fig. 1.
Probiotic count of Lactobacillus acidophilus NCDC 14 and NCDC 16 incorporated sprouted wheat slurry at different time intervals
Fitting the models
The observed values of all the dependent variables along with the level of independent variables for all the probiotic beverage samples are given in Table 1. The pH values for different samples ranged from 4.0 to 4.9, while the acidity of the probiotic beverage samples varied from 0.21 to 0.45 %. The probiotic counts of the different samples were in the range of 8.30 to 10.95 log10 cfu mL−1.
Effect of ingredients’ level on pH
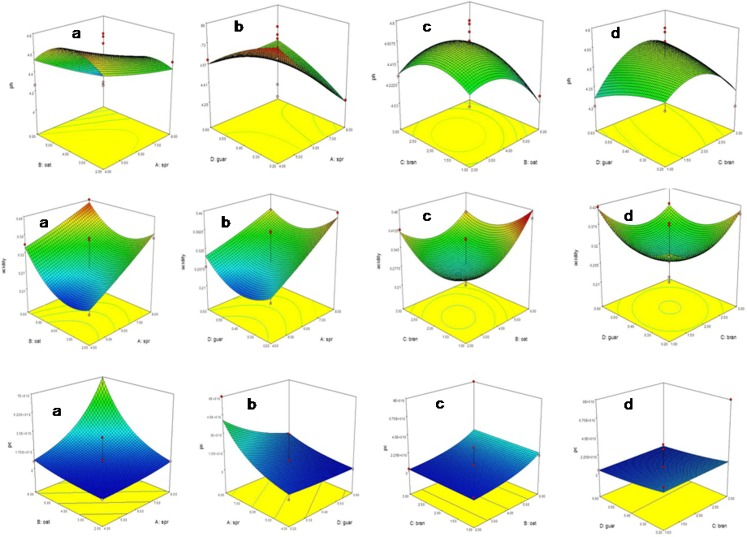
Analysis of variance for the response surface quadratic model for pH is given in Table 2. The F value of 2.309 and p value greater than 0.05 indicate that the model for pH is not significant. It is evident from the results that there is no significant effect of amount of sprouted wheat bran, oat and guar gum on pH values of the probiotic beverage, however, significant effect has been observed by the level of sprouted wheat flour. The response surface graphs for the combined effect of levels of different ingredients on pH are given in 2. The second order polynomial model was obtained by model fitting for pH as the function of sprouted wheat flour, sprouted wheat bran, oat and guar gum as:
 |
where; A-sprouted wheat flour, B- oat, C- sprouted wheat bran and D- guar gum
Table 2.
Analysis of variance for pH of WPB using response surface quadratic model
| Source of variations | SS | df | MS | F value | P value |
|---|---|---|---|---|---|
| Model | 1.195404 | 14 | 0.085386 | 2.309164 | 0.0647ns |
| A | 0.282133 | 1 | 0.282133 | 7.629963 | 0.0153* |
| B | 0.136533 | 1 | 0.136533 | 3.692383 | 0.0753 ns |
| C | 0.012675 | 1 | 0.012675 | 0.34278 | 0.5675 ns |
| D | 7.5E-05 | 1 | 7.5E-05 | 0.002028 | 0.9647 ns |
| AB | 0.0144 | 1 | 0.0144 | 0.389431 | 0.5426 ns |
| AC | 0.0625 | 1 | 0.0625 | 1.690239 | 0.2146 ns |
| AD | 0.1024 | 1 | 0.1024 | 2.769287 | 0.1183 ns |
| BC | 0.024025 | 1 | 0.024025 | 0.649728 | 0.4337 ns |
| BD | 0.164025 | 1 | 0.164025 | 4.435863 | 0.0537 ns |
| CD | 0.04 | 1 | 0.04 | 1.081753 | 0.3159 ns |
| A2 | 0.001558 | 1 | 0.001558 | 0.042145 | 0.8403 ns |
| B2 | 0.214858 | 1 | 0.214858 | 5.810591 | 0.0303* |
| C2 | 0.167614 | 1 | 0.167614 | 4.532935 | 0.0515 ns |
| D2 | 0.00829 | 1 | 0.00829 | 0.224197 | 0.6432 ns |
| Residual | 0.517678 | 14 | 0.036977 | ||
| Lack of Fit | 0.308958 | 10 | 0.030896 | 0.592101 | 0.7716 ns |
| Pure Error | 0.20872 | 4 | 0.05218 | ||
| Correlation Total | 1.713083 | 28 |
WPB wheat based probiotic beverage; SS- sum of squares; df- degree of freedom; MS- mean sum of squares; A-sprouted wheat; B-oat; C-sprouted wheat bran; D-guar gum, *p < 0.05; ns- non significant
Effect of ingredients’ level on acidity
The results of analysis of variance for the response surface quadratic model for acidity are given in Table 3. The R2 (coefficient of determination) value of 0.78 for the model F value of 3.57 implies that the model is significant for acidity. The non-significant lack of fit of the model is desirable. The response surface graphs for the combined effect of different ingredients on acidity are given in Fig. 2. There is significant effect of sprouted wheat flour and oat on the acidity as acidity increases with the increase in sprouted wheat flour and oat concentration. The second order polynomial model was obtained by model fitting for acidity as the function of sprouted wheat flour, sprouted wheat bran, oat and guar gum as:
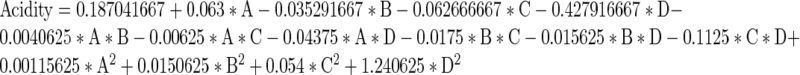 |
where; A-sprouted wheat flour, B- oat, C- sprouted wheat bran and D- guar gum
Table 3.
Analysis of variance for acidity of WPB using response surface quadratic model
| Source of variations | SS | df | MS | F value | P value |
|---|---|---|---|---|---|
| Model | 0.118353 | 14 | 0.008454 | 3.569001 | 0.0117* |
| A | 0.045019 | 1 | 0.045019 | 19.00599 | 0.0007** |
| B | 0.018408 | 1 | 0.018408 | 7.771621 | 0.0145* |
| C | 8.33E-06 | 1 | 8.33E-06 | 0.003518 | 0.9535 |
| D | 0.000102 | 1 | 0.000102 | 0.043097 | 0.8385 |
| AB | 0.001056 | 1 | 0.001056 | 0.445927 | 0.5151 |
| AC | 0.000625 | 1 | 0.000625 | 0.263862 | 0.6155 |
| AD | 0.001225 | 1 | 0.001225 | 0.51717 | 0.4839 |
| BC | 0.0049 | 1 | 0.0049 | 2.06868 | 0.1723 |
| BD | 0.000156 | 1 | 0.000156 | 0.065966 | 0.8010 |
| CD | 0.002025 | 1 | 0.002025 | 0.854913 | 0.3708 |
| A2 | 0.000139 | 1 | 0.000139 | 0.058577 | 0.8123 |
| B2 | 0.023546 | 1 | 0.023546 | 9.940787 | 0.0071** |
| C2 | 0.018915 | 1 | 0.018915 | 7.985354 | 0.0135* |
| D2 | 0.015974 | 1 | 0.015974 | 6.743847 | 0.0211* |
| Residual | 0.033161 | 14 | 0.002369 | ||
| Lack of Fit | 0.006831 | 10 | 0.000683 | 0.103779 | 0.9982ns |
| Pure Error | 0.02633 | 4 | 0.006583 | ||
| Correlation Total | 0.151514 | 28 |
WPB wheat based probiotic beverage; SS- sum of squares; df- degree of freedom; MS- mean sum of squares; A-sprouted wheat; B-oat; C-sprouted wheat bran; D-guar gum, *p < 0.05; **p < 0.01; ns- non significant
Fig. 2.
Response surface curves for combined effect of (a) oat and sprouted wheat flour, (b) guar gum and sprouted wheat flour, (c) bran and oat, (d) guar gum and bran on pH, acidity and probiotic count of probiotic beverage
Effect of ingredients’ level on probiotic count
The response surface linear model was found significant for probiotic count (Table 4) from analysis of variance. The probiotic count of the probiotic beverage samples increased significantly (p < 0.01) with the increasing amount of sprouted wheat flour and oat which showed that oat and sprouted wheat flour exerted a beneficial role in maintaining the probiotic bacteria. Thus, these substrates along with Lactobacillus acidophilus NCDC-14 can be used for the development of wheat based probiotic beverage. The linear model was obtained by model fitting for natural log (ln) probiotic count as the function of sprouted wheat flour, sprouted wheat bran, oat and guar gum as:
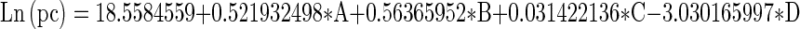 |
where; A-sprouted wheat flour, B- oat, C- sprouted wheat bran and D- guar gum, pc- probiotic count.
Table 4.
Analysis of variance for probiotic count of WPB using response surface linear model
| Source of variations | SS | df | MS | F value | P value |
|---|---|---|---|---|---|
| Model | 32.74519 | 4 | 8.186298 | 6.341315 | 0.0012** |
| A | 13.07585 | 1 | 13.07585 | 10.12889 | 0.0040** |
| B | 15.25018 | 1 | 15.25018 | 11.81318 | 0.0022** |
| C | 0.011848 | 1 | 0.011848 | 0.009178 | 0.9245 ns |
| D | 4.407315 | 1 | 4.407315 | 3.414018 | 0.0770 ns |
| Residual | 30.98272 | 24 | 1.290946 | ||
| Lack of Fit | 21.16977 | 20 | 1.058488 | 0.431466 | 0.9076ns |
| Pure Error | 9.812949 | 4 | 2.453237 | ||
| Correlation Total | 63.72791 | 28 |
WPB wheat based probiotic beverage; SS- sum of squares; df- degree of freedom; MS- mean sum of squares; A-sprouted wheat; B-oat; C-sprouted wheat bran; D-guar gum, **p < 0.01; ns- non significant
The R2 (coefficient of determination) value of 0.514 for the model F value of 6.34 implies that the model is significant for the probiotic count, with a non significant lack of fit suggesting that the model is good. The response surface graphs for combined effect of levels of different ingredients on probiotic count are given in Fig. 2.
Validation based upon desirability
The mean values analyzed through Design expert software for pH, acidity and Natural Logarithm (ln) probiotic count were found to be 4.44, 0.36 % and 22.79, respectively (Table 5). The selected formulations (with desirability >0.87) of probiotic beverages were prepared and evaluated for validating the predicted values. Based upon the validation experiments, the formulation with optimized levels for different ingredients viz. sprouted wheat flour, oat, sprouted wheat bran and guar gum as 7.86, 5.42, 1.42 and 0.6 g, respectively per 100 mL of water was found most suitable for preparation of sprouted wheat and oat based probiotic beverage (Table 6).
Table 5.
Results of response surface models for WPB
| Statistical Parameters | pH | Acidity, % | Probiotic count (ln PC) |
|---|---|---|---|
| Mean | 4.443793 | 0.361724 | 22.79547 |
| Std. Dev. | 0.192294 | 0.048669 | 1.136198 |
| C.V., % | 4.327252 | 13.4547 | 4.984317 |
| PRESS | 2.105725 | 0.080489 | 42.92384 |
| R-Squared | 0.697809 | 0.781134 | 0.513828 |
| Adjusted R-Squared | 0.395618 | 0.562268 | 0.432799 |
| Predicted R-Squared | −0.2292 | 0.46877 | 0.326451 |
| Adequate Precision | 6.146195 | 6.838813 | 9.20421 |
WPB wheat based probiotic beverage; C.V- Coefficient of variation, PRESS- predicted sum of squares
This probiotic beverage contained 86.81 % moisture and 13.19 % total solids, 1.19 % protein, 0.33 % fat, 0.10 % ash, 0.42 % crude fibre, 11.56 % carbohydrates and 54 kcal calories per 100 mL beverage. The iron and calcium content in this probiotic beverage was 1.45 mg and 15.74 mg per 100 mL, respectively. The probiotic count in this beverage was 10.43 log10 cfu mL−1, which was at par with the suggested level of 7 log10 cfu mL−1 (Gomes and Malcata 1999; Shortt 1999). To provide health benefits, the suggested concentration for probiotic bacteria is 6 log10 cfu mL−1 of a product (Lankaputhra and Shah 1995; Saran et al. 2012).
Conclusion
From the two probiotic strains taken for the study, L. acidophilus NCDC-14 was selected for development of sprouted wheat based probiotic beverage as it was more compatible with the substrates. The response surface methodology is a successful tool for optimization. In this study, four ingredients viz. amount of sprouted wheat flour, oat, sprouted wheat bran and guar gum required for maximising the probiotic count were optimized based on response surface methodology. Probiotic count and acidity were significantly affected by the amount of sprouted wheat flour and oat, and maximum probiotic count and acidity were observed at higher concentrations of these ingredients. However, pH was significantly affected by the amount of sprouted wheat flour and other ingredients had a non- significant effect on pH values of the probiotic beverage. The optimum levels of sprouted wheat flour, oat, sprouted wheat bran and guar gum are 7.86, 5.42, 1.42 and 0.6 g respectively per 100 mL of water for the sprouted wheat based probiotic beverage as this formulation resulted in a good probiotic count. Thus, Lactobacillus acidophilus NCDC-14 can be used for the development of potentially probiotic wheat based beverage. The wheat based probiotic beverage (100 ml), prepared using the optimized formulation will provide 13.19 % total solids, 1.19 % protein, 0.33 % fat, 0.10 % ash, 0.42 % crude fibre, 1.45 mg iron, calcium 15.74 mg, 11.56 % carbohydrates, 54 kcal calories and 10.43 log10 cfu mL−1 probiotic count.
Acknowledgments
The authors wish to express sincere thanks to Director, CIPHET for providing different facilities to carry out this work. National Dairy Research Institute, Karnal, India is duly acknowledged for providing the bacterial cultures for conducting the study.
References
- AOAC . Official methods of analysis. 17. Washington, DC: The Association of Official Analytical Chemists; 2000. [Google Scholar]
- Arora S, Jood S, Khetarpaul N. Effect of germination and probiotic fermentation on nutrient composition of barley based food mixtures. Food Chem. 2010;119:779–784. doi: 10.1016/j.foodchem.2009.07.035. [DOI] [Google Scholar]
- Bajad ON, Khedkar CD, Sarode AR, Patil MR, Kalyankar SD, Palange DO. Standardization of a method for preparation of probiotic acido-bifido-yoghurt. J Dairying Foods HS. 2006;25(1):22–27. [Google Scholar]
- Bernardeau M, Guguen M, Vernoux JP. Beneficial lactobacilli in food and feed: long term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiol. 2006;30:487–513. doi: 10.1111/j.1574-6976.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos D, Wang R, Pandiella SS, Webb C. Application of cereals and cereal components in functional foods: a review. Int J Food Micro. 2002;79:131–141. doi: 10.1016/S0168-1605(02)00187-3. [DOI] [PubMed] [Google Scholar]
- Chavan UD, Chavan JK, Kadam SS. Effect of fermentation on soluble proteins and in vitro protein digestibility of sorghum, green gram and sorghum green gram blends. J Food Sci. 1988;53:1574–1575. doi: 10.1111/j.1365-2621.1988.tb09329.x. [DOI] [Google Scholar]
- Erickson KL, Hubbard NE. Probiotic immunomodulation in health and disease. J Nutr. 2000;130:403–409. doi: 10.1093/jn/130.2.403S. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina
- Fuller R. Probiotics: the scientific basis. London: Chapman & Hall; 1992. [Google Scholar]
- Gibson GR, Rastall RA, Fuller R. The health benefits of probiotics and prebiotics. In: Fuller R, Perdigon G, editors. Gut flora, nutrition, immunity and health. 1. Oxford: Blackwell Publishing; 2003. pp. 52–76. [Google Scholar]
- Gomes AMP, Malcata XF. Bifidobacterium ssp. and Lactobacillus acidophilus: biological, biochemical, technological and therapeutical properties relevant for use as probiotics. Trends Food Sci Technol. 1999;10:139–157. doi: 10.1016/S0924-2244(99)00033-3. [DOI] [Google Scholar]
- Holzapfel WH, Haberer P, Geisen R, Bjorkroth J, Schillinger U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr. 2001;73:365S–373S. doi: 10.1093/ajcn/73.2.365s. [DOI] [PubMed] [Google Scholar]
- Jain S, Yadav H, Sinha PR, Naito Y, Marotta F (2008) Dahi containing probiotic lactobacillus acidophilus and lactobacillus casei has a protective effect against salmonella enteritidis infection in mice. Int J Immunopath Pharmacol 21(4) [DOI] [PubMed]
- Lankaputhra WEV, Shah NP. Survival of Lactobacillus acidophilus and Bifidobacterium spp. in the presence of acid and bile salts. Cultured Dairy Pdts J. 1995;30:2–7. [Google Scholar]
- Lorri W, Svanberg U. Lactic-fermented cereal gruels with improved in vitro protein digestibility. Int J Food Sci Nutr. 1993;44:29–36. doi: 10.3109/09637489309017420. [DOI] [Google Scholar]
- Matteuzzia D, Swennena E, Rossia M, Hartmanb T, Lebet V. Prebiotic effects of a wheat germ preparation in human healthy subjects. Food Micro. 2004;21:119–124. doi: 10.1016/S0740-0020(03)00009-1. [DOI] [Google Scholar]
- Pandiyan C, Villi AR, Kumaresan G, Murugan B, Gopalakrishnamurthy TR. Development of synbiotic ice cream incorporating Lactobacillus acidophilus and Saccharomyces boulardii. Int Food Res J. 2012;19(3):1233–1239. [Google Scholar]
- Ranadheera RDCS, Baines SK, Adams MC. Importance of food in probiotic efficacy. Food Res Int. 2010;43:1–7. doi: 10.1016/j.foodres.2009.09.009. [DOI] [Google Scholar]
- Reddy VP, Reddy IS, Christopher MD. Growth pattern of Lactobacillus acidophilus. Tamil Nadu J Vet Anim Sci. 2006;2:142–144. [Google Scholar]
- Saran S, Bisht MS, Singh K, Teotia US. Analyzing probiotic attributes to assess comparatively two isolates of Lactobacillus acidophilus in prebiotics, Honey and Inulin. DHR Int J Biomed Life Sci (DHR-IJBLS) 2012;2(1):26–34. [Google Scholar]
- Shah NP. Probiotic bacteria: selective enumeration and survival in dairy foods. J Dairy Sci. 2000;83:894–907. doi: 10.3168/jds.S0022-0302(00)74953-8. [DOI] [PubMed] [Google Scholar]
- Shah NP. Functional foods: probiotics and prebiotics. Food Technol. 2001;55:46–53. [Google Scholar]
- Sharma M, Mridula D (2013) Probiotics: a comprehensive approach towards health foods. Crit Rev Food Sci Nutr. doi:10.1080/10408398.2011.594185 [DOI] [PubMed]
- Shortt C. The probiotic century: historical and current perspectives. Trends Food Sci Technol. 1999;10:411–417. doi: 10.1016/S0924-2244(00)00035-2. [DOI] [Google Scholar]
- Singh R. Characteristics and technology of traditional cultured Indian milk products. IDF Bull. 2007;415:11–20. [Google Scholar]
- Singh NK, Kumar A, Sinha PR (2007) Chemopreventive effect of acidophilus casei dahi on 1, 2- dimethyl hydrazine induced genotoxicity and preneoplastic lesions during colon carcinogenesis in rats. In proceedings of International Conference on Traditional Dairy Foods, Ndri, Karnal, India 27–28
- Tannock GW. A special fondness for lactobacilli. Appl Environ Microbiol. 2004;70:3189–3194. doi: 10.1128/AEM.70.6.3189-3194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23:62–68. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]