Abstract
Previous studies have demonstrated that combined total sleep deprivation (Wake therapy), sleep phase advance, and bright light therapy (Triple Chronotherapy) produce a rapid and sustained antidepressant effect in acutely depressed individuals. To date no studies have explored the impact of the intervention on unipolar depressed individuals with acute concurrent suicidality. Participants were suicidal inpatients (N=10, Mean age=44±16.4SD, 6F) with unipolar depression. In addition to standard of care, they received open label Triple Chronotherapy. Participants underwent one night of total sleep deprivation (33–36 hours), followed by a three-night sleep phase advance along with four 30-minute sessions of bright light therapy (10,000 lux) each morning. Primary outcome measures included the 17 item Hamilton depression scale (HAM17), and the Columbia Suicide Severity Rating Scale (CSSRS), which were recorded at baseline prior to total sleep deprivation, and at protocol completion on day five. Both HAM17, and CSSRS scores were greatly reduced at the conclusion of the protocol. HAM17 scores dropped from a mean of 24.7±4.2SD at baseline to a mean of 9.4±7.3SD on day five (p=.002) with six of the ten individuals meeting criteria for remission. CSSRS scores dropped from a mean of 19.5±8.5SD at baseline to a mean of 7.2±5.5SD on day five (p=.01). The results of this small pilot trial demonstrate that adjunctive Triple Chronotherapy is feasible and tolerable in acutely suicidal and depressed inpatients. Limitations include a small number of participants, an open label design, and the lack of a comparison group. Randomized controlled studies are needed.
Keywords: Sleep deprivation, Suicidal Ideation, Chronotherapy, Depression, phototherapy, Inpatients
1. Introduction
Major depressive disorder is a neuropsychiatric condition that consists of core symptoms including a persistently depressed mood, anhedonia, sleep disruption, anergia, poor concentration, guilt, hopelessness, appetite changes, and suicidal ideation. Currently there are no commonly used rapid treatments for depression. Suicide is the 10th leading cause of death in the United States, and is even higher among younger individuals between the ages of 10–24, where it is the second leading cause (Heron, 2013). Untreated depression is known to be associated with suicide risk with estimates that 60% of all suicides are associated with inadequately treated depression (Mann et al., 2005). There is an apparent stratified risk of suicide in those who have been admitted to the inpatient unit for depression, with those who have suicidal thoughts, or suicide attempts, posing the highest lifetime risk of committing suicide (Bostwick and Pankratz, 2000). Depression is a major medical issue both domestically and abroad. Depression is the 4th leading cause of disability in the world and has an approximate lifetime prevalence of 16.5% in the United States(Kessler et al., 2003; Murray and Lopez, 1996). Pharmacotherapy, and psychotherapy are the most commonly used treatments but only approximately 67% of non treatment resistant depressed individuals achieve remission with medications or psychotherapy, taking an average of 5–7 weeks to achieve remission in those who find an effective regimen(Rush et al., 2006). Even electroconvulsive therapy (ECT), which is our most dependable, and effective treatment, still takes 2–3 weeks for therapeutic benefit, and has limited availability and cognitive side effects (Sackeim et al., 2007). Although there are promising newer treatments such as repetitive transcranial magnetic stimulation (rTMS) (George et al., 2014) and ketamine (Caddy et al., 2014), there are at this time no commonly used treatments that rapidly treat depression.
Studies have consistently reported a rapid antidepressant response to total sleep deprivation in both unipolar and bipolar depression, first studied by (Pflug and Tolle, 1971), and reviewed extensively by (Wu and Bunney, 1990; Wirz-Justice et al., 2005; and Benedetti et al, 2007). The clinical utility of this technique is limited however, because responders typically relapse rapidly following recovery sleep. The addition of pharmacotherapy (Benedetti et al., 2001; Colombo et al., 2000; Smeraldi et al., 1999; Martiny et al., 2012; Shelton and Loosen, 1993; Szuba et al., 1994; Wu et al., 2009), sleep phase advance (Riemann et al., 1999; Echizenya et al., 2013), and bright light therapy(Echizenya et al., 2013; Martiny et al., 2012; Martiny et al., 2013; Neumeister et al., 1996; Wu et al., 2009) to sleep deprivation have each demonstrated efficacy in preventing some individuals from relapsing into depression. Some early studies have reported that combined total sleep deprivation, sleep phase advance, and bright light therapy, dubbed Triple Chronotherapy, along with concomitant pharmacotherapy, produces a rapid improvement in depressive symptoms which endures for as long as 9 weeks (Echizenya et al., 2013; Martiny et al., 2012; Wu et al., 2009). If the early, encouraging results of Triple Chronotherapy hold up to further study, the technique represents a near ideal inpatient treatment, as it is inexpensive, relatively easy to carry out, and has minimal side effects.
Despite encouraging early results, only one published report has attempted to use triple chronotherapy in suicidal patients, and in that trial only bipolar depressed patients were included. That study used a slightly different variation of chronotherapy that included three nights of sleep deprivation every other night with three light therapy sessions, combined with lithium (Benedetti et al., 2014). The lack of data utilizing Triple Chronotherapy in acutely suicidal patients significantly limits its utility in the United States, where few non-suicidal patients are admitted to the inpatient unit. Furthermore, published trials to this point have excluded those with comorbid illness, which also limits the clinical usefulness of this intervention to a minority of patients. We subsequently sought to determine if adjunctive triple chronotherapy was safe and feasible in acutely depressed and suicidal inpatients.
2. Materials and Methods
2.1 Participants
We included participants with non-psychotic unipolar, or bipolar depression (who were on a therapeutic dose of a mood stabilizer), age 18–75. We excluded patients who were in a mixed state, had active psychosis, had active panic disorder, were actively withdrawing from a substance of abuse, had a history of seizures, or had active unstable medical or neurologic illness.
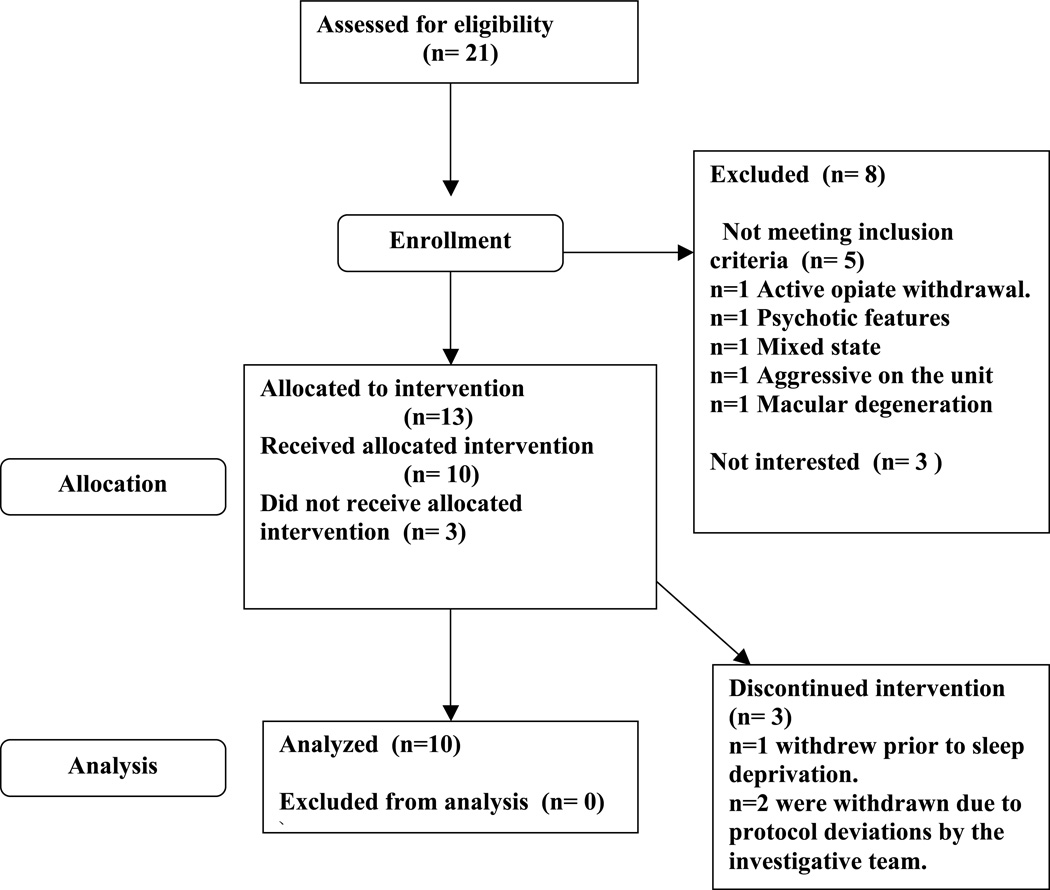
We recruited participants from inpatient units at the Medical University of South Carolina (MUSC) Institute of Psychiatry (IOP) during the months of October 2013–March 2014 after referral from the treating inpatient team. Inpatient teams first briefly explained the chronotherapy intervention to patients who were admitted. Study team members then met with interested patients to obtain informed consent. All interested patients who met inclusion criteria, and did not meet exclusion criteria were included in the study. A total of 21 potential participants were referred, with three not being interested in the study, and four meeting exclusion criteria. Of the remaining referrals, 14 signed written informed consent, one of which later failed initial screening. The remaining sample of 13 enrolled in the below described protocol which was approved by the MUSC intuitional review board (IRB). Of the included participants one participant withdrew from the study prior to the first sleep deprivation and stated they were no longer wanting to participate, and two others were excluded from data analysis due to protocol deviations related to the investigative team, leaving a final sample of 10. Of the two that were withdrawn, our team missed awakening the first following the first recovery sleep night, and our team placed the second in an excessively noisy room during the first recovery night of sleep, and they were unable to sleep (They have a diagnoses of bipolar type I, and following two sleepless nights we thought the risk of manic switch outweighed any possible therapeutic benefit of continuing the protocol) (Figure 1). The mean age of participants was 44±16.4, six of whom were women, and none of whom had bipolar depression (Table 1). All but one participant carried comorbid Axis I, or Axis II diagnosis, which consisted of the following: Five participants met criteria for generalized anxiety disorder, four participants met criteria for dysthymia, three participants met criteria for borderline personality disorder, three participants met criteria for post traumatic stress disorder, three met criteria for alcohol dependence in early remission, two met criteria for social anxiety disorder, and one met criteria for opiate dependence in early remission. We initially recruited two participants with a diagnosis of bipolar disorder, however both of those participants were excluded from analysis due to protocol deviations as described above. Only three participants were initially admitted for a suicide attempt, while all patients were admitted for suicidal ideation.
Figure 1.
Screening and Enrollment Flowchart
Table 1.
Demographics
| N=10 | |
|---|---|
| Age | Mean=44±16.4SD |
| Gender | 4 Male, 6 Female |
| Race/Ethnicity | 9 (90%) Caucasian 1 (10%) African American |
| Duration of Current Episode | 15.4 months±13.2SD |
| Number of Lifetime Episodes | 7.8±5.8SD |
| Number of Failed Medications/ECT |
5.5±5.7SD One failure of ECT |
This was an adjunctive procedure, and with the exception of holding hypnotics on the night of sleep deprivation, all standard of care pharmacotherapy was allowed. In addition to pharmacotherapy, all patients on the unit received milieu therapy, group therapy, and social work interventions. The group was heterogeneous as far as treatment resistance. The group had an average of 5.5±5.7 medications that were either failed or were not tolerated. One participant previously failed ECT. The medications that were either started or continued by the treating team were as follows: All participants were on antidepressants during the study; five were on serotonin selective reuptake inhibitors (SSRI)’s, two were on serotonin non-selective reuptake inhibitors (SNRI)’s, four were on trazodone, three were on mirtazapine, one was on vilazodone, one was on phenelzine, two were on cytomel, three were on benzodiazepines, one was on quetiapine, one was on gabapentin, one was on belladonna, and one was on melatonin. Prior to, or during the weeklong protocol, the following medications were started, or titrated: One had an SNRI titrated, one had phenelzine started, three had mirtazapine started or titrated, two had titrations of an SSRI, one had cytomel started, one had quetiapine started, one had prazosin started, one had a benzodiazepine started, and one had gabapentin started.
2.2 Triple Chronotherapy procedure
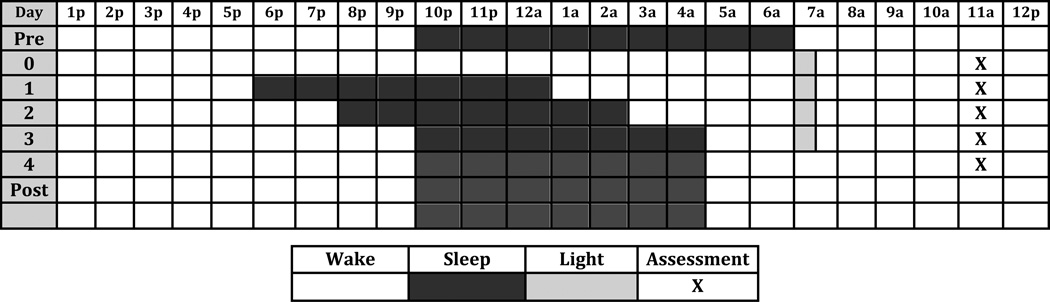
The Triple Chronotherapy procedure we used closely resembled the one described in the manual written by Wirz-Justice at al (Wirz-Justice et al., 2013). Recruited participants filled out the Morningness-Eveningness questionnaire (MEQ) (Horne and Ostberg, 1976), which has been shown to predict optimal light therapy timing. Participants then underwent one night of total sleep deprivation (Day 0), followed by a three day sleep phase advance (Sleep occurred between 6pm, and 1am on day 1, 8pm and 3am on day 2, and 10pm and 5am on day 3). In addition they received bright light therapy on the mornings of days 1, 2, 3, and 4, for 30 minutes at 10,000lux, with the timing set by MEQ. The start time of light exposure varied between 6am and 8am. Following the four-day intervention, participants were asked to remain on as close a schedule as possible to 10pm to 5am (although this was not monitored as we considered the intervention to have been completed). We did recommend that they expand their time in bed to a maximum of 8 hours if they were experiencing daytime sleepiness with 7 hours in bed. Light therapy was generally discontinued, however participants were offered recommendations on the appropriate use of light therapy if they desired to continue it once they left the hospital. (Figure 2)
Figure 2.
2.3 Data collection
On the day proceeding total sleep deprivation (Day 0), after participants signed informed consent, we performed a chart review as well as reviewed pertinent laboratory testing. We then performed a MINI neuropsychiatric examination(Sheehan et al., 1998) to confirm diagnoses, administered a 17-Item Hamilton depression rating scale (HAMD17)(Hamilton, 1960), Columbia Suicide Severity Rating Scale (CSSRS)(Posner et al., 2011), and a Young Mania Rating scale (YMRS)(Young et al., 1978), inquiring about the previous 7 days. In addition, we collected self report measures at baseline, including the Inventory of Depressive Symptoms Self Report (IDS-SR)(Rush et al., 1996), the Patient Health Questionaire-9 (PHQ9)(Spitzer et al., 1999), the Epworth Sleepiness scale (ESS)(Johns, 1993), the Pittsburgh Sleep Quality Index (PSQI)(Buysse et al., 1989), and the Scale for Suicidal Ideation (SSI)(Beck et al., 1979). On the day following total sleep deprivation, and the days following the first two nights of sleep phase advance (Days 1–3), we administered the 6-item version of the Hamilton Depression Rating Scale (HAM-D6)(O'Sullivan et al., 1997), the CSSRS, the YMRS, the IDS-SR, the ESS, and the SSI all asking about the previous day only. On the day following the third sleep phase advance night (Day4), we administered the HAM17, CSSRS, and YMRS, and collected the IDS-SR, ESS, SSI, PHQ9, and PSQI asking about the previous day only. We defined remission from depression as a HAM17 score of less than 7. We defined response from either depression (HAM17), or suicidal ideation (CSSRS) as a greater than 50% drop in score from baseline.
2.4 Data analysis
Because of the small sample size (n=10), we chose to use more valid and more conservative non-parametric statistical tests. We used Wilcoxon’s signed rank test to evaluate the change in outcome measures throughout the four-day study follow-up. For the HAMD-17, we compared day 4 to baseline. For other measures collected on each day, we made pairwise comparisons of the change since baseline for each day of follow-up. We used SAS Enterprise version 4.3 for all analyses (SAS Institute, Inc., Cary NC).
3. Results
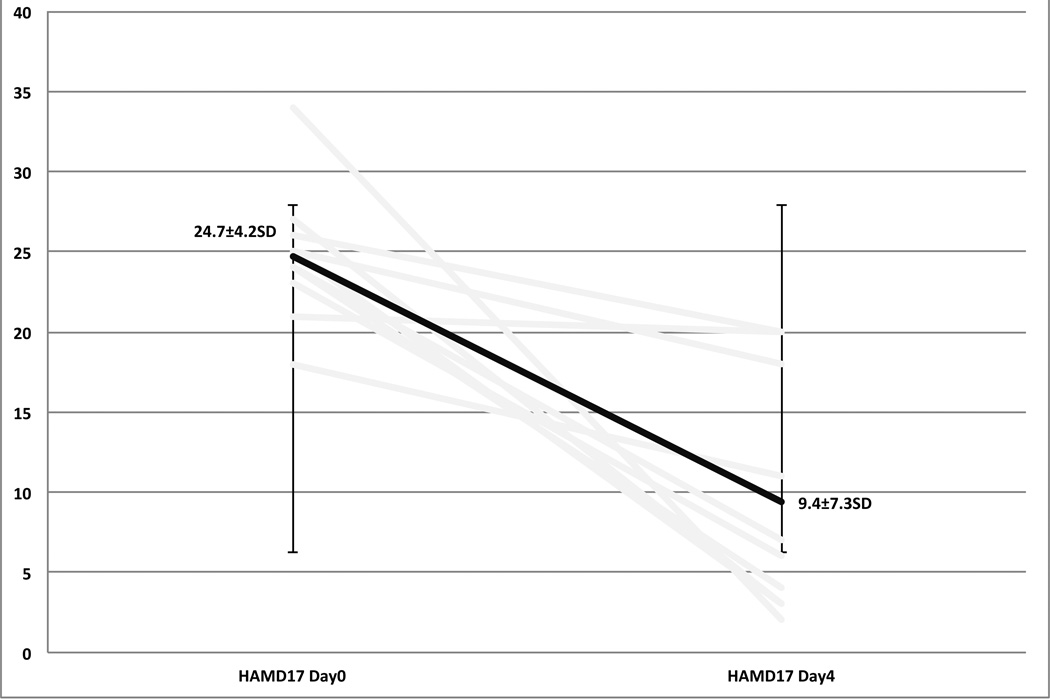
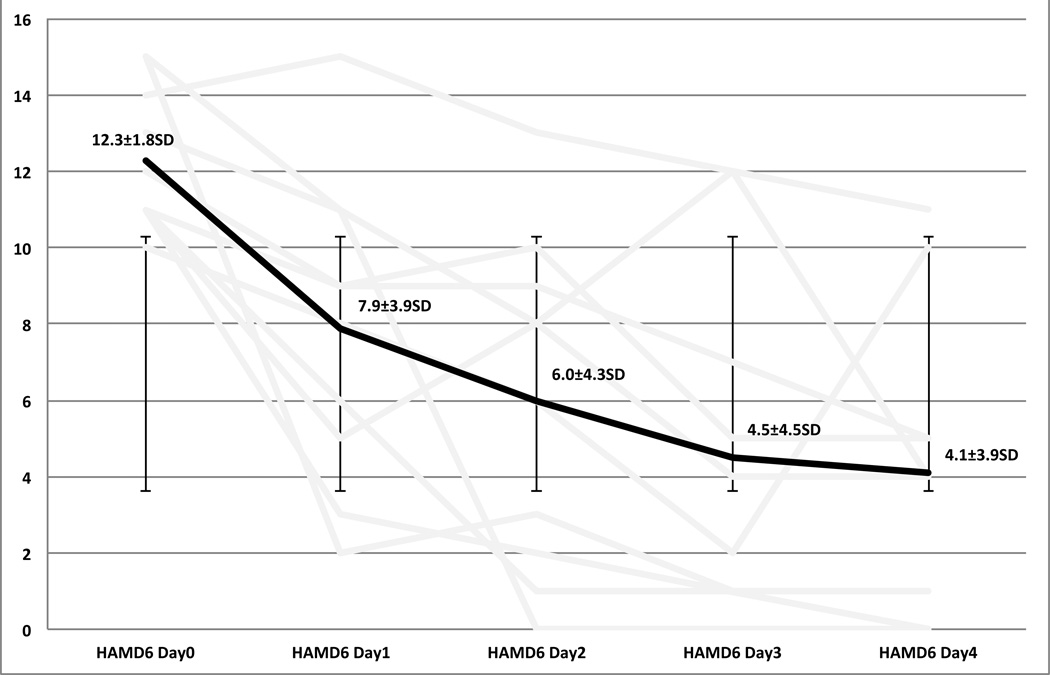
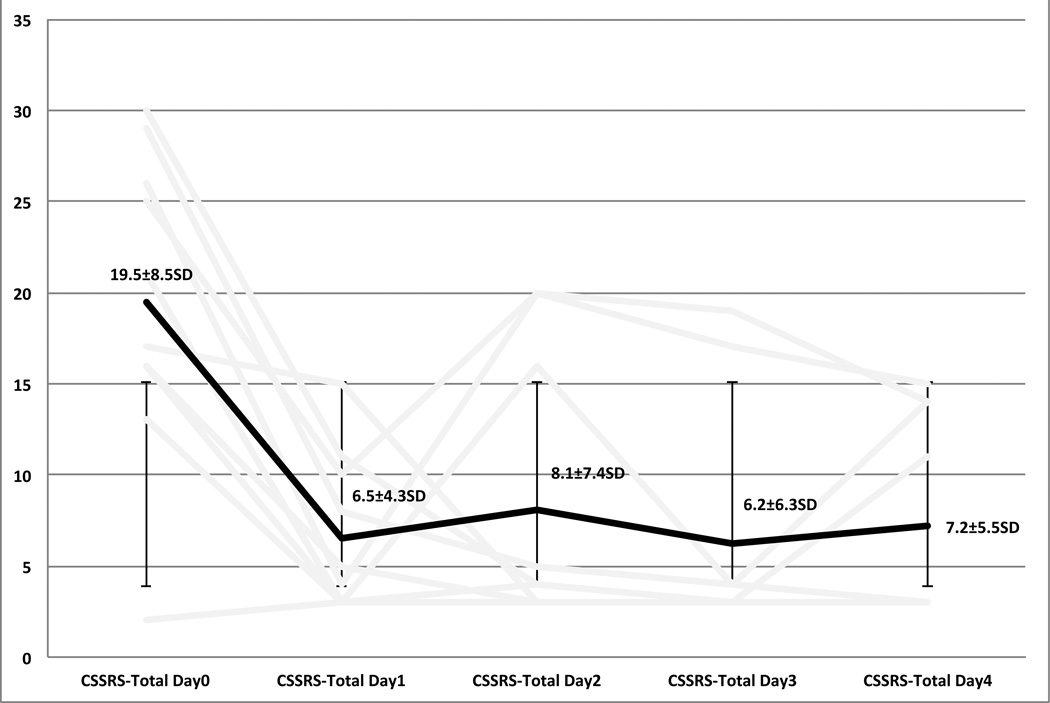
As compared to baseline there was a statistically and clinically significant decrease in both clinician, and self rated scales of depression and suicidal ideation. The 17-Item Hamilton depression scale went from an average of 24.7±4.2SD at day 0, to a final score of 9.4±7.3SD on day 4. Six out of ten participants met criteria for remission on the Ham17 (60%). Sixty-percent met criteria for response (Figures 3, 4). The Columbia Suicide Severity Index went from an average of 19.5±8.5SD on day 0 to a final score of 7.2±5.5SD on day 4. Sixty percent of participants met criteria for a response on the CSSRS (Figure 5). Self report measures were also collected each day, having been filled out while receiving light therapy, and can be found in table 2.
Figure 3.
Hamilton Depression RaBng Scale 17-Item
Figure 4.
Hamilton Depression RaDng Scale 6-Item
Figure 5.
Columbia Suicide Severity RaEng Scale Total
Table 2.
Measures Score (± Standard Deviation)
| Measure | Baseline | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|---|
| HAM-D (17) | 24.7(±4.2) | 9.4(±7.3) p=.002 |
|||
| HAM-D (6) | 12.3(± 1.8) | 7.9(±3.9) p<.004 |
6(± 4.3) p=.002 |
4.5(± 4.5) p=.002 |
4.1(± 3.9) p<.004 |
| CSSRS | 19.5(±8.5) | 6.5(±4.3) p<.004 |
8.1(± 7.4) p<.01 |
6.2(± 6.3) p<.004 |
7.2(± 5.5) p<.01 |
| IDS | 52.1(±6.7) | 45.4 (±11.5) p=.07 |
39.1(±16.8) p=.06 |
25.9(±13.5) p<.004 |
22.3(±14) p<.004 |
| SSI | 17.2(±9.4) | 15(±9.8) | 12.2(±10.5) p<.02 |
8.5(±8.0) p<.004 |
7.4(±7.9) p<.004 |
HAM-D, 17-Item and 6-Item Hamilton Rating Scale for Depression; CSSRS, Columbia-Suicide Severity Rating Scale; IDS, Inventory of Depressive Symptomatology; SSI, Scale of Suicidal Ideation.
4. Discussion
This small, open label pilot study suggests that adjunctive Triple Chronotherapy is safe and tolerable in acutely suicidal, unipolar depressed inpatients. These results complement and extend the recently published study demonstrating safety of another variant of Chronotherapy in suicidal Bipolar Depressed inpatients (Benedetti et al., 2014). This conclusion, along with any conclusion regarding treatment efficacy, must however be made in the context of significant experimental limitations, with special attention made to the small sample size, significant medication changes, time in the structured hospital environment, and the lack of a control group.
It is possible that this small cohort of participants would have improved even more rapidly and robustly with treatment as usual, or that the response observed was directly the result of treatment as usual, a placebo response, or some combination of the two. This is particularly true considering all patients were on concurrent pharmacotherapy, and receiving group therapy on the unit. However, typically neither pharmacotherapy, nor group psychotherapy has an onset of action that is as rapid as was observed in our cohort. Furthermore, comparison trials of Chronotherapy in the setting of medication use demonstrate that groups receiving both Chronotherapy and medications have a more rapid, and robust improvement as compared to groups receiving either alone, and response and remission rates have been consistent with our results (Benedetti et al., 2001; Colombo et al., 2000; Shelton and Loosen, 1993; Szuba et al., 1994).
It is of note that Chronotherapy was well tolerated by all participants who agreed to the procedure. Most participants reported only transient sleepiness, which was most prominent between the hours of 3am and 6am on the night of total sleep deprivation, and following the first night of recovery sleep. There was a small statistically non-significant (p=0.74) increase in suicidal ideation on day 2 compared to day 1, however this small increase was mostly accounted for by our non-responders, was still considerably below reported suicidal ideation prior to starting the procedure, and not likely to be clinically significant. Only three participants were withdrawn from the study, one of which withdrew before beginning total sleep deprivation, and the other two of which had to be withdrawn due to easily correctible study team errors (One we did not correctly wake up, and the other we placed in a room that was too close to unit activity).
A further limitation of this study that is particularly noteworthy is the lack of follow-up of our cohort after hospital discharge (Due to loss to follow-up). Historic data would suggest the possibility of rapid relapse (Wu and Bunney, 1990), however recent trials have demonstrated durability of the antidepressant effect of combined chronotherapeutic interventions with medications (Benedetti et al., 2005; Echizenya et al., 2013; Martiny et al., 2012; Wu et al., 2009).
After consideration of the above significant limitations, the results found in this pilot study still expand the potential clinical group that can undergo this intervention. Given the large effect size, ease of administration, mild side effects, and inexpensive nature of this intervention, further study is warranted. Although our findings in the context of the other literature in the field are very encouraging, we believe further study must take place prior to widespread clinical adoption. The two areas of study that are most lacking are data comparing active chronotherapy to an adequate active sham condition, and further durability data. Controlled trials would ideally include both an active sham group, and a treatment as usual group. An active sham could include partial sleep deprivation (Depriving the first part of the nights sleep), a three-day sleep phase delay, and placebo light therapy. All such sham interventions have been utilized previously and have been demonstrated to be safe. Such a sham group could control for placebo effects related to undergoing a procedure, the added staff attention included in sleep deprivation, and a rigid sleep wake schedule. Including a treatment as usual group would control for improvement based upon pharmacotherapy, psychotherapy, and the structured hospital environment.
4.1 Conclusion
Based upon the results of our small open label pilot study, Triple Chronotherapy is safe and feasible to administer in acutely depressed and suicidal inpatients. Further work is needed to determine treatment effects in a placebo-controlled trial.
-
-
Few rapid treatments exist for the treatment of depression.
-
-
Triple Chronotherapy shows promise as a rapidly acting adjunct antidepressant.
-
-
Despite its promise, Triple Chronotherapy lacks safety data in suicidal patients.
-
-
We found the use of Triple Chronotherapy is safe in depressed suicidal inpatients.
Acknowledgements
We would like to thank the acute inpatient team at the Institute of Psychiatry of the Medical University of South Carolina for their tireless effort. Without your help this study could not have occurred. We would also like to thank the Drug Abuse Research Track (DART) program at the Medical University of South Carolina with special thanks to Drs.Back and Brady, along with the grant that supports it (NIDA R25 DA020537-06 (PI’s Back and Brady).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office).
He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from Gregory Sahlem Sahlem@musc.edu
Author Contributions:
Gregory L. Sahlem was the Primary investigator of the study. He contributed in experimental design, regulatory procedures, data collection, data analysis, and manuscript preparation.
Benjamin Kalivas contributed in experimental design, data analysis, and manuscript preparation.
James B. Fox contributed in recruitment, and manuscript preparation.
Kayla Lamb contributed to regulatory procedures, and manuscript preparation.
Amanda Roper contributed in experimental design, and manuscript preparation.
Emily N. Williams contributed in experimental design, recruitment, and manuscript preparation.
Nolan R. Williams contributed in experimental design, and manuscript preparation.
Jeffrey E. Korte was the statistician on the project. He contributed in data analysis and manuscript preparation.
Zachary D. Zuschlag contributed in recruitment and manuscript preparation.
Salim El Sabbagh contributed in recruitment and manuscript preparation Constance Guille was a mentor for the project and contributed in experimental design and manuscript preparation.
Kelly S. Barth was a mentor for the project and contributed in recruitment and manuscript preparation.
Thomas W. Uhde was a mentor for the project and contributed in experimental design, and manuscript preparation.
Mark S. George was a mentor for the project. He contributed in experimental design, regulatory procedures, data analysis, and manuscript preparation.
E.Baron Short was a mentor for the project. He contributed in experimental design, regulatory procedures, data analysis, and manuscript preparation.
Contributor Information
Benjamin Kalivas, Email: Kalivas@musc.edu.
James B. Fox, Email: foxj@musc.edu.
Kayla Lamb, Email: lamb@musc.edu.
Amanda Roper, Email: pearcea@musc.edu.
Emily N. Williams, Email: emilynicolewilliams@gmail.com.
Nolan R. Williams, Email: nolrywillia@gmail.com.
Jeffrey E. Korte, Email: korte@musc.edu.
Zachary D. Zuschlag, Email: zuschlag@musc.edu.
Salim El Sabbagh, Email: elsabbag@musc.edu.
Constance Guille, Email: Guille@musc.edu.
Kelly S. Barth, Email: Stephen@musc.edu.
Thomas W. Uhde, Email: uhdepsych@musc.edu.
Mark S. George, Email: georgem@musc.edu.
E.Baron Short, Email: shorteb@musc.edu.
References
- Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. Journal of consulting and clinical psychology. 1979;47:343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Barbini B, Campori E, Fulgosi MC, Pontiggia A, Colombo C. Sleep phase advance and lithium to sustain the antidepressant effect of total sleep deprivation in bipolar depression: new findings supporting the internal coincidence model? Journal of psychiatric research. 2001;35:323–329. doi: 10.1016/s0022-3956(01)00034-6. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Barbini B, Fulgosi MC, Colombo C, Dallaspezia S, Pontiggia A, et al. Combined total sleep deprivation and light therapy in the treatment of drug-resistant bipolar depression: acute response and long-term remission rates. The Journal of clinical psychiatry. 2005;66:1535–1540. doi: 10.4088/jcp.v66n1207. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Barbini B, Colombo C, Smeraldi E. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007;11:509–522. doi: 10.1016/j.smrv.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Riccaboni R, Locatelli C, Poletti S, Dallaspezia S, Colombo C. Rapid treatment response of suicidal symptoms to lithium, sleep deprivation, and light therapy (chronotherapeutics) in drug-resistant bipolar depression. The Journal of clinical psychiatry. 2014;75(2):133–140. doi: 10.4088/JCP.13m08455. [DOI] [PubMed] [Google Scholar]
- Bostwick MP, Pankratz VS. Affective Disorders and Suicide Risk: A Reexamination. The American journal of psychiatry. 2000:1925–1932. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Caddy C, Giaroli G, White TP, Shergill SS, Tracy DK. Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Therapeutic Advances in Psychopharmacology. 2014;4:75–99. doi: 10.1177/2045125313507739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo C, Lucca A, Benedetti F, Barbini B, Campori E, Smeraldi E. Total sleep deprivation combined with lithium and light therapy in the treatment of bipolar depression: replication of main effects and interaction. Psychiatry research. 2000;95:43–53. doi: 10.1016/s0165-1781(00)00164-5. [DOI] [PubMed] [Google Scholar]
- Echizenya M, Suda H, Takeshima M, Inomata Y, Shimizu T. Total sleep deprivation followed by sleep phase advance and bright light therapy in drug-resistant mood disorders. Journal of affective disorders. 2013;144:28–33. doi: 10.1016/j.jad.2012.06.022. [DOI] [PubMed] [Google Scholar]
- George MS, Raman R, Benedek DM, Pelic CG, Grammer GG, Stokes KT, et al. A Two-site Pilot Randomized 3 Day Trial of High Dose Left Prefrontal Repetitive Transcranial Magnetic Stimulation (rTMS) for Suicidal Inpatients. Brain Stimul. 2014;7(3):421–431. doi: 10.1016/j.brs.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M. Deaths: Leading Causes for 2010. National Vital Statistics Reports. 2013;62 [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International journal of chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCSR) JAMA : the journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Apter A, Bertolote J, Beautrais A, Currier D, Haas A, et al. Suicide prevention strategies: a systematic review. JAMA : the journal of the American Medical Association. 2005;294:2064–2074. doi: 10.1001/jama.294.16.2064. [DOI] [PubMed] [Google Scholar]
- Martiny K, Refsgaard E, Lund V, Lunde M, Sorensen L, Thougaard B, et al. A 9-week randomized trial comparing a chronotherapeutic intervention (wake and light therapy) to exercise in major depressive disorder patients treated with duloxetine. The Journal of clinical psychiatry. 2012;73:1234–1242. doi: 10.4088/JCP.11m07625. [DOI] [PubMed] [Google Scholar]
- Martiny K, Refsgaard E, Lund V, Lunde M, Sorensen L, Thougaard B, et al. The day-to-day acute effect of wake therapy in patients with major depression using the HAM-D6 as primary outcome measure: results from a randomised controlled trial. PloS one. 2013;8:e67264. doi: 10.1371/journal.pone.0067264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Goessler R, Lucht M, Kapitany T, Bamas C, Kasper S. Bright light therapy stabilizes the antidepressant effect of partial sleep deprivation. Biological psychiatry. 1996;39:16–21. doi: 10.1016/0006-3223(95)00086-0. [DOI] [PubMed] [Google Scholar]
- O'Sullivan RL, Fava M, Agustin C, Baer L, Rosenbaum JF. Sensitivity of the six-item Hamilton Depression Rating Scale. Acta psychiatrica Scandinavica. 1997;95:379–384. doi: 10.1111/j.1600-0447.1997.tb09649.x. [DOI] [PubMed] [Google Scholar]
- Pflug B, Tolle R. Disturbance of the 24-hour rhythm in endogenous depression and the treatment of endogenous depression by sleep deprivation. International pharmacopsychiatry. 1971;6:187–196. doi: 10.1159/000468269. [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia–Suicide Severity Rating Scale: Initial Validity and Internal Consistency Findings From Three Multisite Studies With Adolescents and Adults. The American journal of psychiatry. 2011:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann D, Konig A, Hohagen F, Kiemen A, Voderholzer U, Backhaus J, et al. How to preserve the antidepressive effect of sleep deprivation: A comparison of sleep phase advance and sleep phase delay. European archives of psychiatry and clinical neuroscience. 1999;249:231–237. doi: 10.1007/s004060050092. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:244–254. doi: 10.1038/sj.npp.1301180. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- Shelton RC, Loosen PT. Sleep deprivation accelerates the response to nortriptyline. Progress in neuro-psychopharmacology & biological psychiatry. 1993;17:113–123. doi: 10.1016/0278-5846(93)90036-r. [DOI] [PubMed] [Google Scholar]
- Smeraldi E, Benedetti F, Barbini B, Campori E, Colombo C. Sustained Antidepressant Effect of Sleep Deprivation Combined with Pindolol in Bipolar Depression: A Placebo-controlled Trial. Neuropsychopharmacology. 1999;20(4):380–385. doi: 10.1016/S0893-133X(98)00129-8. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA : the journal of the American Medical Association. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Szuba MP, Baxter LR, Jr, Altshuler LL, Allen EM, Guze BH, Schwartz JM, et al. Lithium sustains the acute antidepressant effects of sleep deprivation: preliminary findings from a controlled study. Psychiatry research. 1994;51:283–295. doi: 10.1016/0165-1781(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, Berger M, Lam RW, Martiny K, Terman M, et al. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35:939–944. doi: 10.1017/s003329170500437x. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for affective disorders : a clinician's manual for light and wake therapy. 2nd, rev. ed. Basel: Karger; 2013. [Google Scholar]
- Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. The American journal of psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- Wu JC, Kelsoe JR, Schachat C, Bunney BG, DeModena A, Golshan S, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biological psychiatry. 2009;66:298–301. doi: 10.1016/j.biopsych.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry : the journal of mental science. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]