Figure 2.
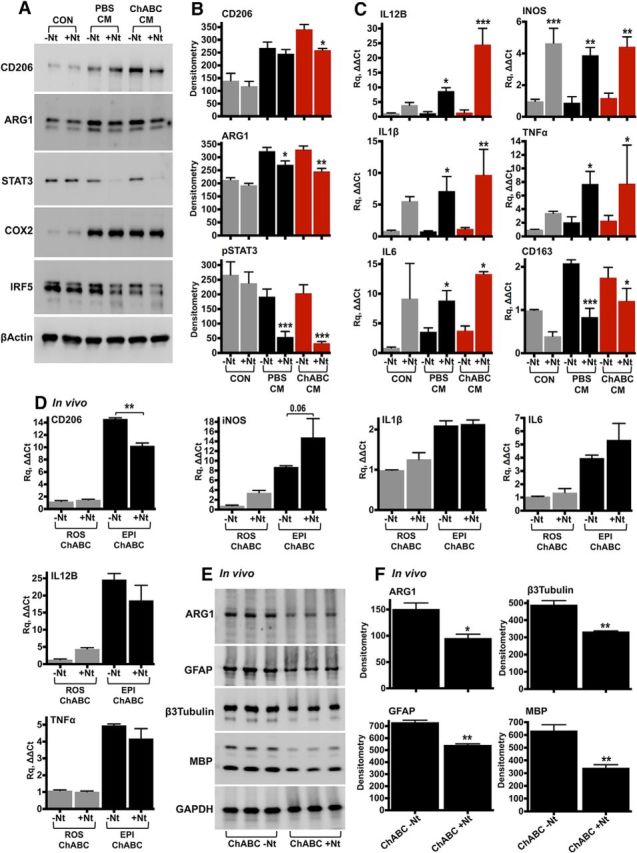
IL-10 neutralization in vitro and in vivo. A, B, Rat peritoneal macrophages were stimulated for 24 h with CM derived from the injury epicenter (T10) of either PBS- or ChABC-injected spinal cords. IL-10 was neutralized in the conditioned medium using anti-IL-10 antibodies (+Nt, 250 ng). Control cells (CON) were cultured in serum-free DMEM, supplemented with either isotype rabbit IgG (−Nt) or anti-IL-10 antibodies. Protein levels of inflammatory markers were assessed by immunoblotting (A) and were quantified by densitometry (B). Results are reported as the mean ± SEM, n = 3–4 experiments. β-Actin served as the loading control. C, The effect of IL-10 neutralization on the expression of inflammatory genes was assessed using TaqMan quantitative PCR in peritoneal macrophages stimulated with conditioned medium for 12 h. The fold change in gene expression was calculated relative to unstimulated cells (CON, −Nt). GAPDH served as the housekeeping gene. Results are the mean ± SEM, n = 3–4 independent experiments. For B and C, ANOVA and pairwise comparisons with Fisher's least significant difference test (−Nt vs +Nt). *p < 0.05, **p < 0.01, ***p < 0.001. D–F, For in vivo neutralization of IL-10, T10 contused rats received intrathecal injections of ChABC (0.5 U/injection) supplemented with either 5 μg of isotype rabbit IgG (ChABC, −Nt) or 5 μg of anti-IL-10 neutralizing antibodies (ChABC, +Nt). CD206, iNOS, IL-1β, IL-6, IL-12B, and TNF-α expression was measured by quantitative PCR in the injury epicenter (EPI) and compared with intact rostral tissue (ROS) 7 d after SCI (D). n = 3 animals per group, ANOVA and Fisher's least significant difference test (−Nt vs +Nt). **p < 0.01. E, F, ARG1, GFAP, β3-tubulin, and MBP were examined in injured spinal cord protein extracts with immunoblotting (E) and were quantified by densitometry (F). Unpaired t test, *p < 0.05, **p < 0.01. GAPDH served as the loading control.
