Abstract
Pathologic and physiologic factors acting on the heart can produce consistent pressure changes, volume overload, or increased cardiac output. These changes may then lead to cardiac remodeling, ultimately resulting in cardiac hypertrophy. Exercise can also induce hypertrophy, primarily physiologic in nature. To determine the mechanisms responsible for each type of remodeling, it is important to examine the heart at the functional unit, the cardiomyocyte. Tests of individual cardiomyocyte function in vitro provide deeper understanding of the changes occurring within the heart during hypertrophy. Examination of cardiomyocyte function during exercise primarily follows one of two pathways: the addition of hypertrophic inducing agents in vitro to normal cardiomyocytes, or the use of trained animal models and isolating cells following the development of hypertrophy in vivo. Due to the short lifespan of adult cardiomyocytes, a proportionately scant amount of research exists involving the direct stimulation of cells in vitro to induce hypertrophy. These attempts provide the only current evidence, as it is difficult to gather extensive data demonstrating cell growth as a result of in vitro physical stimulation. Researchers have created ways to combine skeletal myocytes with cardiomyocytes to produce functional muscle cells used to repair pathologic heart tissue, but continue to struggle with the short lifespan of these cells. While there have been promising findings regarding the mechanisms that surround cardiac hypertrophy in vitro, the translation of in vitro findings to in vivo function is not consistent. Therefore, the focus of this review is to highlight recent studies that have investigated the effect of exercise on the heart, both in vitro and in vivo.
Keywords: myocyte, heart, exercise, hypertrophy, signaling mechanisms
INTRODUCTION
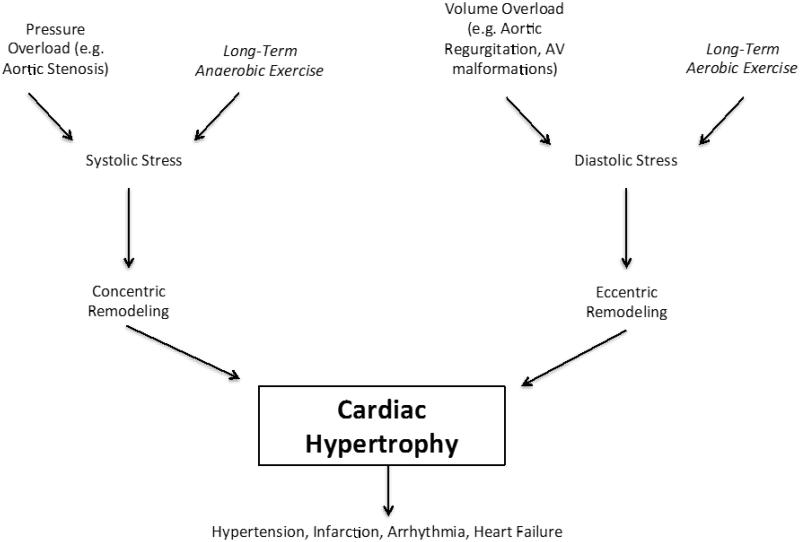
Cardiac hypertrophy is defined as an increase in heart mass in response to an increased load on the myocardium attributable to physiologic or pathologic stimulation (Cooper, 1987). Pathological conditions, including diabetes, hypertension, and myocardial infarction, can increase the width or length of individual cardiomyocytes, leading to gross changes in ventricular structure. Interestingly, certain hypertrophic changes observed in diseased states are mimicked by exercise training, however, these are physiologic in nature (D'Andrea et al., 2007). Hypertrophy, regardless of mechanism, may lead to hypertension, infarction, or arrhythmia, ultimately predisposing individuals to heart failure (Gaasch and Zile, 2004). This fact underlies the importance of understanding and differentiating the mechanisms of physiologic- and pathologic-induced hypertrophy (D'Andrea, Caso, 2007, Gielen et al., 2010). Figure 1 provides a visual representation of the gross similarities and differences in these mechanisms.
Cardiomyocyte growth occurs in either eccentric or concentric forms, depending on the type of stimulus (Fernandes et al., 2011). Volume overload causes cardiomyocytes to grow eccentrically through serial sarcomere addition, increasing myocardial mass and chamber volume. Concentric hypertrophy, a result of parallel addition of sarcomeres in the cardiomyocyte, increases heart wall thickness without increasing chamber volume, creating greater pressure output (D'Andrea, Caso, 2007, Fernandes, Soci, 2011). Endurance-based aerobic exercise training requires consistent blood flow to skeletal muscle, thus favoring an eccentric building mechanism to meet the increased volume demands. Long-term anaerobic exercise leads to vasoconstriction, resulting in concentric hypertrophy to meet increasing pressure demands on the heart.
Similar concentric and eccentric mechanisms of remodeling are observed in pathologic episodes. Conditions such as hypertension, aortic stenosis, and other stimuli causing pressure overload and increased systolic wall stress, instigate concentric remodeling of the heart (Bernardo et al., 2010). Pathologic conditions, such as aortic regurgitation and arteriovenous fistulas, increase diastolic wall stress, leading to volume overload and result in eccentric hypertrophy (Bernardo, Weeks, 2010). Hypertrophic adaptation provides the greatest compensation to sustain heart function in response to pathologic or physiologic insult. Although both forms of remodeling can be associated with negative long-term outcomes, eccentric remodeling poses greater risks for cardiac injury.
A robust, reproducible in vitro model of hypertrophy in isolated cardiomyocytes is essential to understand the mechanisms of hypertrophy (Wang et al., 2010). Such a model could differentiate cardiomyocyte hypertrophy that occurs in the myocardium as a syncytium from separate processes that occur in individual cells. Furthermore, myocyte mechanotransduction in vivo becomes problematic due to the ability of the cardiomyocyte to sense mechanical load and generate mechanical force, ensuring the survival of the organism (Russell et al., 2010). These problems have forced researchers to mimic in vivo studies in freshly isolated cardiomyocytes. Although in vitro methods have their own inherent issues, including the contamination of nonmuscle cells and absence of cell-to-cell communication, they possess certain advantages over in vivo methods. Specifically, in vitro methods allow for mechanical load and chemical stress on myocyte signal transduction and growth to be studied in the absence of other cofounding variables (Garlie et al., 2011, Russell, Curtis, 2010).
In vitro cardiomyocyte remodeling can provide a deeper knowledge required for developing therapeutic treatments. The following section provides an overview of studies investigating pathologic and physiologic cardiac hypertrophy specific to in vitro cell functionality, in order to justify in vivo findings. We will categorically review physical and chemical hypertrophic triggers while highlighting certain therapeutic options. Additionally, we will discuss the possibility of skeletal muscle surrogates for cardiomyocyte modeling.
Physical Triggers
Stress Sensing
Cardiac mechanoreceptors detect alterations in both pressure and volume, signaling changes in ventricular diameter and heart wall thickness (Blaauw et al., 2010). The varied forms and locations of stress sensors in the myocardium allow for gross communication, enabling detection of changes in individual cell sizes. Several cardiomyocyte organelles and protein structures potentially participate in stretch sensing with numerous participating mechanisms (Blaauw, van Nieuwenhoven, 2010). The sarcolemma modulates intracellular calcium (Ca2+) concentration and senses mechanical action while proteins such as integrin, melusin, and integrin-linked kinase transmit mechanical signals to the cytoskeleton. Specific tyrosine kinases and Z-disc proteins, including titin, are sensors of stretch and cardiomyocyte deformation, respectively (Blaauw, van Nieuwenhoven, 2010). Recently, Methawasin et al. (Methawasin et al., 2014) showed reduced diastolic chamber stiffness but depressed end-systolic elastance when upregulating compliant titins. A higher prevalence of the beneficial effects was observed upon exercised conditions. These cellular stretch receptors, regardless of signaling pathway, respond by enhancing protein synthesis, cell volume, cell surface area, and expression of the brain natriuretic peptide (BNP) hypertrophic marker.
Integrins/Mechanical Stress
Recent work has described cardiomyocyte mechanoreceptor properties and hypothesized that integrins, specifically β1-integrin existing predominantly as the β1D spliced isoform, are prime candidates for acting as biomechanical sensors considering their location at the junction of the extracellular matrix and Z-disc (Lal et al., 2007). Animal models have demonstrated the relationship between integrins and cardiac hypertrophy. Increased expression of β1-integrin in rats was directly correlated to a higher expression of hypertrophic genes than in knockout β1-integrin mice. Using stretch in neonatal ventricular myocytes from rats, researchers could replicate the hypertrophic nature created through gene expression. Mechanical stretch activates mitogen-activated protein (MAP) kinases independent of the angiotensin II type I (AT1) receptor that mediates hypertrophy and regulates β1 (Lal, Verma, 2007). A lack of β1-integrin expression in cardiomyocytes leads to fibrosis and dilated cardiomyopathy within six months of age (Brancaccio et al., 2006). Transgenic mice die perinatally and display diffuse fibrotic replacement when a β1 mutant disrupts the β1 integrin under the control of the α1 myosin heavy chain promoter (Brancaccio, Hirsch, 2006). These data support the fact that integrins act as important sensors of mechanical stretch to cardiac tissue and suggests a role for integrins in cardiac remodeling following insult.
Angiotensin II type I Receptor
The angiotensin II type 1 (AT1) receptor plays a crucial role in the development of load-induced cardiac hypertrophy; furthermore, mechanical stress has been found to induce AT1 receptor activation independent of angiotensin II (ANG-II) involvement. Yasuda et al. (Yasuda et al., 2008) showed that increased stretch alone causes a conformational change in the AT1 receptor in a model using cultured human embryonic kidney (HEK) 293 cells. Addition of the ANG-II receptor blocker (ARB), candesartan, resulted in the inhibition of mechanical stress-induced AT1 receptor activation, as well as the prevention of pressure-induced hypertrophy in ANG-II-null mice (Yasuda, Miura, 2008). Though these findings are apparent in HEK293 cells, differing evidence exists in experiments utilizing different animal models. Data from cultured adult rabbit cardiomyocytes showed that stretch-induced hypertrophy (mechanical stress) did not involve any autocrine/paracrine actions of ANG-II, TGF-β1, or IGF-I (Blaauw, van Nieuwenhoven, 2010). Results indicated that candesartan and irbesartan did not prevent stretch-induced hypertrophy. Thus, the specific pathway or receptor that induces hypertrophy in an isolated cell remains unclear. Furthermore, adult cardiomyocytes that respond to growth factors may not respond to ANG-II as the maturation process shifts away from neonatal distinctions with continued development (Blaauw, van Nieuwenhoven, 2010). Further elucidation of the mechanisms of AT1 receptor-induced hypertrophy from mechanical stress was demonstrated through the calcineurin pathway (Zhou et al., 2010). Cultured neonatal mouse myocytes subjected to mechanical stress exhibited cardiac hypertrophy independent of the ANG-II pathway through the AT1 receptor. Moreover, calcineurin was shown to be a vital pathway for hypertrophy through the AT1 receptor-mediated ANG-II independent response (Zhou, Li, 2010).
Specific physical stimuli and individual chemical pathways may determine whether the hypertrophy is pathologic or physiologic (Russell, Curtis, 2010). Exercise and postnatal growth can trigger the release of insulin growth factor-1 (IGF-1) to compensate for the added stress on the heart during exertion or development. In contrast, pathologic stimuli, such as pressure overload, will cause elevated levels of ANG-II, catecholamines, and endothelin-1 (ET-1) (Bernardo, Weeks, 2010). These studies suggest that remodeling is a dynamic process with numerous mechanisms and factors contributing to its progression.
Chemical Triggers
CT-1/JAK/STAT
A number of chemical triggers can contribute to the progression of cardiac hypertrophy. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway transduces signals from extracellular ligands found on cytokines, growth factors, and hormones to the nucleus to create the appropriate cellular response (Barry et al., 2007). During acute cardiac pressure overload, the respective JAK/STAT pathway sub-groups are activated to facilitate the necessary hypertrophic compensatory response. Simvastatin (SIM) inhibited the effects of cardiac hypertrophy via the JAK/STAT pathway, denoting the importance of the JAK/STAT pathway in the hypertrophic response (Barry, Townsend, 2007).
Cardiotrophin-1 (CT-1), a cytokine related to interleukin-6 and an activator of the JAK/STAT pathway, can induce cardiac hypertrophy in vitro following incubation with isolated cardiomyocytes (Liu et al., 2008). This study also suggests that SIM alleviates responses induced by CT-1 via the JAK/STAT pathway in cultured cardiomyocytes (Liu, Shen, 2008). Cell surface area and protein content were obtained to assess the amount of hypertrophy. The experiment successfully demonstrated an attenuation of hypertrophy in cells treated with CT-1, attributable to increased SIM levels (Liu, Shen, 2008). Together, these data indicate that the JAK/STAT signal transduction pathway plays a crucial role in cardiac hypertrophy, possibly due to its ability to recognize prominent chemical and hormonal signals that are up regulated during pathologic or physiologic insult.
lmcd1/Dyxin
The Z-disc plays an important role in cardiomyocyte contraction and viability, as it is the location of the T-tubule and the site of exchange for numerous substrates. Researchers have investigated pathways associated with G-protein-coupled receptors and their ligands (ANG-II, ENDO-II, catecholamines), focusing on the function of Z-discs in sarcomeres (Frank et al., 2010). Genomic screening comparing gene expression of either biomechanical stretch or pharmacological stimulation with phenylephrine, has identified lmcd1/Dyxin as a primary contributor to hypertrophy (Frank, Frauen, 2010). Imcd1/Dyxin cardiomyocyte overexpression and knockout studies indicated that Icmd1/Dyxin may potentially alter the molecular architecture of the Z-disc by disrupting inhibitors and blunting pathologic hypertrophy. Additionally, lmcd1/Dyxin shares its subcellular localization with a portion of the calcineurin pool in the cardiomyocyte Z-disc. Research has shown that calcineurin levels directly correlate to the pathologic hypertrophic response (Frank, Frauen, 2010). Further studies utilizing the knockout Imcd1/Dyxin model could lead to the development of a new drug targeting lmcd1/Dyxin, with the ability to stunt the progression of cardiac hypertrophy associated with heart disease.
Renin angiotensin system (RAS)
High-density lipoproteins (HDL), well known for their role in reverse cholesterol transport, may inhibit the renin angiotensin system (RAS) and suppress cardiac hypertrophy. The RAS is the primary regulator for many physiological processes of the cardiovascular system, and alterations in its function may be involved with cardiac hypertrophy (Lin et al., 2011). ANG-II, a primary component of the RAS, controls vasoconstriction, hypertrophy, fibrosis, and blood pressure (Lin, Gong, 2011). Cardiac hypertrophy can be inhibited partially by the inhibition of the angiotensin II type 1 receptor (AT1-R). In single cell cultured cardiomyocytes, the addition of HDL with ANG-II demonstrated that HDL inhibited hypertrophic responses induced by ANG-II (Lin, Gong, 2011). By measuring the rate of protein synthesis and using RT-PCR, researchers demonstrated ANG-II inhibition with HDL treatment, postulating that the underlying mechanism for the effects of HDL are due to inhibition of inflammatory neutrophil recruitment and cardiomyocyte apoptosis (Lin, Gong, 2011). Thus, the RAS plays an important role in the development of cardiac hypertrophy as demonstrated by the inhibition of RAS by HDL and the subsequent blunted hypertrophic response.
Reactive oxygen species (ROS)/Allicin
Numerous studies have explored the relationship between cardiac hypertrophy and ROS created in the heart through ANG-II, phenylephrine (PE), endothelin (ET-1), TNF-α, and mechanical processes (Dai et al., 2011); (Ceylan-Isik et al., 2013); (Gan et al., 2005); (Heineke and Molkentin, 2006); (McCain and Parker, 2011)). Allicin, a garlic metabolite, effectively inhibits ROS generated through the previously mentioned routes (Gomez-Cabrera et al., 2008b). When placed in culture with ANG-II treated cardiomyocytes, allicin reduced expression of 3H-leucine, a marker of protein synthesis, as well as the overall size of the myocyte (Gomez-Cabrera, Domenech, 2008b). Although ROS may cause damage to the cardiomyocyte, it could be essential for the survival and regulation of cellular mechanisms. One study stated that ROS produced from contracting myocytes provides the signal for an increase in the expression of enzymes that aid in the cell’s adaptation to exercise (Gomez-Cabrera et al., 2008a). Further research is required to more fully understand the dichotomy between the advantageous and disadvantageous properties of ROS, specifically exploring its short-term role during exercise and the local response to increased stress.
Ras/Raf/ERK1/2 pathway
Mitofusin-2 (Mfn2), a transmembrane GTPase embedded in the outer membrane of the mitochondria and endoplasmic reticulum, inhibits the Ras/Raf/ERK1/2 pathway (Fang et al., 2007). A number of studies demonstrated a relationship between inhibition of this pathway and the development of cardiac hypertrophy. One study stimulated cultured neonatal cardiomyocytes overnight, showing that stimulation leads to the down regulation of Mfn2, increased myocyte surface area, and increased 3H-Leucine (Fang, Moore, 2007). Together, these data imply that the down regulation of Mfn2 leads to protein synthesis, a precursor of cardiac hypertrophy. Although further studies are needed regarding Mfn2 interaction in cardiac hypertrophy, the down regulation of Mfn2 could be linked to pathologic and physiologic cardiac hypertrophy.
Pimentel et al. (Pimentel et al., 2006) observed that mechanical strain caused hypertrophy via ROS dependent, post-translational modification of Ras. These modifications ultimately led to the activation of the Raf/Mek/Erk growth pathway (Pimentel, Adachi, 2006). The subsequent increase in Ras activity was halted by the overexpression of the N17 dominant negative mutant of Ras, inhibiting protein synthesis and Erk activation, and the adenoviral overexpression of catalase (Pimentel, Adachi, 2006). These findings demonstrate the importance of the Ras/Raf/ERK pathway in regulating cardiac hypertrophy, while also presenting a potential target of either ROS or prevention of Mfn2 inhibition.
Peroxisome proliferator-activated receptor-α signaling pathway (PPAR-α)
The peroxisome proliferator-activated receptor-α signaling pathway inhibits cardiac hypertrophy through activation of adenosine monophosphate-activated protein kinase (AMPK) (Rimbaud et al., 2009a). Cultured neonatal myocytes were stimulated with phenylephrine and the AMPK activating drug, 5-aminoimidazole 1-carboximide ribonucleoside (AICAR). Up regulation of the PPAR-α pathway by AICAR suppressed the hypertrophic effects of phenylephrine. Additionally, activation of AMPK by AICAR reduced leucine incorporation, expression of ANP and β-MHC mRNA, and significantly suppressed cardiac hypertrophy (Rimbaud et al., 2009b). These findings suggest that increases in PPAR-α limit pathologic cardiac hypertrophy.
In contrast, another study explained that PPAR-α levels rise as a result of physiologic hypertrophy (Rimbaud, Sanchez, 2009b). While both studies utilized rats for the animal model, the age groups differed significantly. The AICAR study used neonatal rats from one to three days old, but the exercise study used seven week-old adults. The age separation may affect certain metabolic properties of the heart and could also represent a temporal difference in the mechanism of hypertrophy. These studies, when viewed together, represent the synergistic effects of pathways and their ability to force the heart to adapt in the most appropriate fashion. When the heart is subjected to a pathologic insult, evidence suggests that the down regulation of PPAR-α occurs, creating a pro-hypertrophic environment; however, if an activator of the PPAR-α pathway is increased, hypertrophy will decrease (Rimbaud, Sanchez, 2009b). Exercise trained hearts exhibit an increase in PPAR-α levels during physiologic-induced hypertrophy. Therefore, it is likely that increases in PPAR-α during pathologic insults may adjust the heart to develop a more physiologic-based, compensatory hypertrophy to sustain proper function.
Pathologic insults were demonstrated by showing that tumor-bearing mice presented with reduced levels of PPAR-α and carnitine palmitoyltransferase (CPT1β), compared to those without tumors (Tian et al., 2011). This phenomenon suggested that pathological hypertrophy occurs through the down regulation of fatty acid metabolism. These data also provide another application for the inhibition of the PPAR-α pathway that could be beneficial in sustaining proper heart function. An additional experiment investigated the anti-hypertrophic effects of GW0742, a specific agonist of PPAR-β/δ, on ANG II-induced hypertrophic cardiomyocytes (Sheng et al., 2008). This study used neonatal Wistar rat ventricular cardiomyocytes and demonstrated attenuation of hypertrophy through PPAR-β/δ activation by a decrease in myocyte surface area, protein synthesis, atrial naturietic peptide (ANP), and brain naturietic peptide (BNP) expression (Sheng, Ye, 2008). By inhibiting specific inflammatory makers, GW0742 blocked hypertrophic events. This evidence strongly suggests that PPAR-α and its associated regulators are crucial modulators of cardiomyocyte hypertrophy.
AVP and ANP
The essential hormone, [Arg8]]-vasopressin (AVP), produced by the posterior pituitary, has primarily been recognized for its role in osmotic regulation. Broadly, the vasopressin receptor V1b works in the anterior pituitary affecting corticotropin release (Stewart et al., 2008). The vasopressin receptor V2 has an antidiuretic role in the kidney (Jard et al., 1986, Thibonnier et al., 1994). V1a receptors located on smooth muscle regulate vasoconstriction, while those on cardiac muscle cells modulate cardiac function (Hiroyama et al., 2007). The latter function has prompted detailed studies of the receptor. Hypertrophy in cultured neonatal mouse cardiomyocytes has demonstrated an involvement of the vasopressin receptor, V1a. AVP can activate vasopressin receptors V1a, V1b and V2, but it was discovered that only V1a is expressed in mouse neonates (Hiroyama, Wang, 2007). Studies utilizing the receptor knockout mouse showed that AVP is implicated in the stimulation of myocardial cell hypertrophy through enhancing protein synthesis and cellular growth without affecting cell division (Hiroyama, Wang, 2007). Additionally, an increase in ANP, a marker of hypertrophy highly expressed in the adult atria, was found in the ventricular cardiomyocytes. Therefore, ANP may also play a role in the development of hypertrophy in neonates due to its high secretion during enlargement (Hiroyama, Wang, 2007). ANP is also secreted as a result of the mechanical stress on the cardiomyocytes, correlating with the trigger of hypertrophy. Lastly, the addition of AVP to cardiomyocytes increased the phosphorylation of ERK1/2, resulting in activation of the Ras/Raf/ERK1/2 and indicating the pathway through which AVP causes hypertrophy.
microRNA regulation
MicroRNA (miR) downregulation has been observed in studies of mouse and human models of cardiac hypertrophy, particularly with miR-133 and miR-1 (Caré et al., 2007). Overexpression of these miRs demonstrated an inhibitory effect of cardiac hypertrophy in vitro. As miRs are also known to suppress tumor development, their overexpression may help improve the function of cardiomyocytes affected by tumor growth. Another key finding was decreased expression of miR-133 and miR-1 in the following pathological or physiologic hypertrophic models: transverse aortic arch-constricted mice (TAC), transgenic (Tg) mice with selective cardiac overexpression of a constitutively active mutant of Akt kinase and exercised rats (Caré, Catalucci, 2007). A model of inducing in vitro hypertrophy by adding phenylephrine or endothelin-1 to cultured neonatal mouse myocytes was utilized (Caré, Catalucci, 2007). When combining hypertrophy with the miR-133 precursor Ad133, the cultured cardiomyocytes showed decreased cell size, protein synthesis, and fetal genes (Caré, Catalucci, 2007).
Researchers confirmed the significant role of miRs in cardiac hypertrophy. Not only did they agree with the suppression of hypertrophy from miR-133 and miR-1, but they also discovered that in pathological thoracic aortic-banded (TAB) mice, miR-21, miR-23a, and miR-125b were increased in both agonist-induced hypertrophic cardiomyocytes and in TAB-induced hypertrophic hearts (Tatsuguchi et al., 2007). Interestingly, in vitro work showed a relatively low expression of these miRs in neonatal cardiomyocytes, which were unchanged during hypertrophy (Tatsuguchi, Seok, 2007). Other studies demonstrated that miR-21 inhibition led to the activation of apoptosis and decreased cell proliferation (Tatsuguchi, Seok, 2007). It appears that expression of miRs changes according to the type of miR, the stimuli acting on the cardiomyocytes, the model of animal, and the environment pertaining to either in vitro or in vivo experiments.
In Vitro Hypertrophy
The majority of the aforementioned cultured cardiomyocyte experiments used rat neonatal ventricular myocytes due to their ability to divide once and remain viable for up to one week (Berry et al., 2007, Birla et al., 2008). The aim of this review has been to identify possible mechanisms identified in in vivo studies that could be applied to establish in vitro models. Beta-adrenergic agonists, such as isoproterenol, trigger an increase in cardiomyocyte size and protein content with gene expression similar to in vivo models (Berry, Naseem, 2007). Similarly, phenylephrine and ANG-II have been used to increase the size of cardiomyocytes as a hypertrophic model for in vitro cardiac hypertrophy (Berry, Naseem, 2007). It has been popular to assess the growth and function of neonatal rat ventricular cardiomyocytes (NRVMs) through stimulation software programs that can record velocity, amplitude and calcium decay of individual myocytes (Berry, Naseem, 2007).
There is reluctance to attempt these experiments in adult rat ventricular myocytes due to their inability to survive and remain functional in culture for extended periods of time. However, there are instances of adult cell usage including an experiment using adult cardiomyocytes isolated from wild type and transgenic rats (Jeong et al., 2006). An increase was found in transgenic peak shortening of the myocyte by 89% compared to the wild type. Cells were transfected with either AdLacZ or AdPICOT to determine the effects of PICOT (PKC-Interacting cousin of Thioredoxin), a protein kinase C (PKC) inhibitor, on cardiomyocytes. The AdPICOT cells showed an 84% increase in percent shortening (indicative of cell function), a 57% increase in maximal rate of contraction (-dL/dt), and an 83% increase in the maximal rate of relaxation (+dL/dt) when compared to AdLacZ transfected cells (Jeong, Cha, 2006). It is speculated that PICOT activity in failing hearts may reverse hypertrophic conditions and restore contractility (Jeong, Cha, 2006). Additionally, PICOT is thought to enhance the inotropic properties of cardiomyocytes. These experiments demonstrated that methods exist and have been implemented to create hypertrophy in cardiomyocytes in vitro. The mechanisms previously described in this review could be used to make these in vitro findings more robust.
Myotubes/Skeletal Muscle
Unlike cardiomyocyte in vitro studies, there is an abundance of information regarding the underlying mechanisms of in vitro skeletal muscle hypertrophy (Fujita et al., 2007, Nedachi et al., 2008, Nikolić et al., 2012, Serrano et al., 2008). Due to the terminal nature and lack of regeneration in cardiac myocytes, it is easier to obtain data from highly regenerative lines of skeletal myocytes, particularly when they develop into myotubes. Data exist regarding in vitro exercise in skeletal myocytes, however, extensive research currently uses skeletal muscle cells in an attempt to further develop cardiac muscle repair solutions (Pedrotty et al., 2008, Reinecke et al., 2000, Schuldt et al., 2008). If successful implantation of skeletal muscle tissue into the myocardium occurs, this could open up the possibility of using other muscle cell lines combined with functional heart cells to relay information regarding adaptation and plasticity of heart function. Skeletal muscle cells have been identified as having the highest potential in achieving a connection with cardiomyocytes (Schuldt, Rosen, 2008). Although their action potential is less than one tenth of that of a cardiomyocyte, they possess similar longitudinal contraction and twitches (Schuldt, Rosen, 2008). Even though skeletal muscle cells show similar attributes to cardiomyocytes, there have been problems associated with sustaining the connection between the two different cells. Certain clinical trials displayed subtle improvements in patients with implanted skeletal muscle cells, however, larger populations showed trends toward the development of tachyarrhythmias, suggesting the differentiation of skeletal muscle cells into cardiac muscle cells has yet to be perfected (Schuldt, Rosen, 2008).
One way skeletal cells increase mitochondrial biogenesis and endurance output is through the up regulation of biomarkers, such as PPARγ, coactivator-1α (PGC-1α) and sirtuin 1 (SIRT1), by natural flavonoids including quercetin (Gurd et al., 2009). If present in skeletal muscle, quercetin may affect mitochondria production in cardiac muscle, demonstrating the ability for longer endurance capacity and possible eccentric hypertrophy. In contrast, another study showed that SIRT1 is not responsible for an increase in oxidative capacity within tissue models. These findings oppose most cell line models, which maintain that PGC-1α is still responsible for oxidative capacity (Gurd, Yoshida, 2009). Another group stated that PGC-1α is enriched in heart tissue due to a need for high oxygen capacity (Schilling and Kelly, 2011), adding that development of a PGC-1/ERR axis would regulate energy metabolism and mitochondrial function in the heart (Schilling and Kelly, 2011). Regarding pathologic hypertrophy, this axis could serve as a therapeutic tool to monitor and regulate proper heart function, possibly suppressing disease-induced hypertrophy. Further study investigating the in vitro expression of PGC-1α and utilizing methods to control the PGC-1/ERR axis could aid in understanding the mechanisms associated with in vivo disease regulation.
CONCLUSIONS
Complete understanding of cardiac hypertrophy requires substantial knowledge of the processes that affect the single heart cell. The myriad of intricate pathways make it difficult for in vivo studies, including echocardiography, to provide the fullest and most accurate data (Hoshijima, 2006). Therefore, development and utilization of cardiomyocyte models of hypertrophy have produced promising results. Studies of pathway triggers like JAK/STAT, PPAR-α, G-coupled proteins lmcd1 and dyxin, encompassing RAS processes, ROS suppression, or hypertrophy associated hormones like AVP and ANP demonstrate a greater ability to investigate hypertrophy when viewed in vivo. Studies demonstrating the use of skeletal muscle cells in connection with cardiomyocytes also present innovative options for aiding in pathologic cardiac hypertrophy suppression, particularly alleviating fibrotic tissue (Schuldt, Rosen, 2008). The functional success of combining skeletal muscle cells with cardiac muscle cells may further elucidate the importance of cell-to-cell interaction versus individual cell survival. This could lead to possible cardiac repair methods to replace detrimental fibrotic tissue. Preliminary studies utilizing cardiomyocytes in vitro demonstrate that these models are possible and could be further elaborated with the stimuli discussed in this review.
ACKNOWLEDGMENTS
Funding was provided in part by Bennett Memorial Scholarships from the College of Medicine at the Ohio State University to MCI and TDN and grants #NIH R01NR012618 and R01ES019923 to LEW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE:
None
REFERENCES
- Barry SP, Townsend Pa, Latchman DS, Stephanou A. Role of the JAK-STAT pathway in myocardial injury. Trends in molecular medicine. 2007;13:82–9. doi: 10.1016/j.molmed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacology & therapeutics. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Berry JM, Naseem RH, Rothermel Ba, Hill Ja. Models of cardiac hypertrophy and transition to heart failure. Drug Discovery Today: Disease Models. 2007;4:197–206. [Google Scholar]
- Birla RK, Dow DE, Huang Y-C, Migneco F, Khait L, Borschel GH, et al. Methodology for the formation of functional, cell-based cardiac pressure generation constructs in vitro. In vitro cellular & developmental biology Animal. 2008;44:340–50. doi: 10.1007/s11626-008-9098-9. [DOI] [PubMed] [Google Scholar]
- Blaauw E, van Nieuwenhoven Fa, Willemsen P, Delhaas T, Prinzen FW, Snoeckx LH, et al. Stretch-induced hypertrophy of isolated adult rabbit cardiomyocytes. American journal of physiology Heart and circulatory physiology. 2010;299:H780–7. doi: 10.1152/ajpheart.00822.2009. [DOI] [PubMed] [Google Scholar]
- Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovascular research. 2006;70:422–33. doi: 10.1016/j.cardiores.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Caré A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, et al. MicroRNA-133 controls cardiac hypertrophy. Nature medicine. 2007;13:613–8. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- Ceylan-Isik AF, Dong M, Zhang Y, Dong F, Turdi S, Nair S, et al. Cardiomyocyte-specific deletion of endothelin receptor A rescues aging-associated cardiac hypertrophy and contractile dysfunction: role of autophagy. Basic Res Cardiol. 2013;108:335. doi: 10.1007/s00395-013-0335-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cooper Gt. Cardiocyte adaptation to chronically altered load. Annual review of physiology. 1987;49:501–18. doi: 10.1146/annurev.ph.49.030187.002441. [DOI] [PubMed] [Google Scholar]
- D'Andrea A, Caso P, Scarafile R, Salerno G, De Corato G, Mita C, et al. Biventricular myocardial adaptation to different training protocols in competitive master athletes. International Journal of Cardiology. 2007;115:342–9. doi: 10.1016/j.ijcard.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–46. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Moore X-L, Gao X-M, Dart AM, Lim YL, Du X-J. Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life sciences. 2007;80:2154–60. doi: 10.1016/j.lfs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Fernandes T, Soci UPR, Oliveira EM. Eccentric and concentric cardiac hypertrophy induced by exercise training: microRNAs and molecular determinants. Brazilian Journal of Medical and Biological Research. 2011;44:836–47. doi: 10.1590/s0100-879x2011007500112. [DOI] [PubMed] [Google Scholar]
- Frank D, Frauen R, Hanselmann C, Kuhn C, Will R, Gantenberg J, et al. Lmcd1/Dyxin, a novel Z-disc associated LIM protein, mediates cardiac hypertrophy in vitro and in vivo. Journal of molecular and cellular cardiology. 2010;49:673–82. doi: 10.1016/j.yjmcc.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Fujita H, Nedachi T, Kanzaki M. Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Experimental cell research. 2007;313:1853–65. doi: 10.1016/j.yexcr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Gaasch WH, Zile MR. Left ventricular diastolic dysfunction and diastolic heart failure. Annu Rev Med. 2004;55:373–94. doi: 10.1146/annurev.med.55.091902.104417. [DOI] [PubMed] [Google Scholar]
- Gan XT, Rajapurohitam V, Haist JV, Chidiac P, Cook MA, Karmazyn M. Inhibition of phenylephrine-induced cardiomyocyte hypertrophy by activation of multiple adenosine receptor subtypes. The Journal of pharmacology and experimental therapeutics. 2005;312:27–34. doi: 10.1124/jpet.104.073122. [DOI] [PubMed] [Google Scholar]
- Garlie JB, Hamid T, Gu Y, Ismahil MA, Chandrasekar B, Prabhu SD. Tumor necrosis factor receptor 2 signaling limits β-adrenergic receptor-mediated cardiac hypertrophy in vivo. Basic research in cardiology. 2011;106:1193–205. doi: 10.1007/s00395-011-0196-6. [DOI] [PubMed] [Google Scholar]
- Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122:1221–38. doi: 10.1161/CIRCULATIONAHA.110.939959. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera M-C, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free radical biology & medicine. 2008a;44:126–31. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008b;44:126–31. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Gurd BJ, Yoshida Y, Lally J, Holloway GP, Bonen A. The deacetylase enzyme SIRT1 is not associated with oxidative capacity in rat heart and skeletal muscle and its overexpression reduces mitochondrial biogenesis. The Journal of physiology. 2009;587:1817–28. doi: 10.1113/jphysiol.2008.168096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nature reviews Molecular cell biology. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- Hiroyama M, Wang S, Aoyagi T, Oikawa R, Sanbe A, Takeo S, et al. Vasopressin promotes cardiomyocyte hypertrophy via the vasopressin V1A receptor in neonatal mice. European journal of pharmacology. 2007;559:89–97. doi: 10.1016/j.ejphar.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. American journal of physiology Heart and circulatory physiology. 2006;290:H1313–25. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jard S, Gaillard R, Guillon G, Marie J, Shoenenberg P, Muller A, et al. Vasopressin antagonists allow demonstration of a novel type of vasopressin receptor in the rat adenohypophysis. Molecular Pharmacology. 1986;30:171–7. [PubMed] [Google Scholar]
- Jeong D, Cha H, Kim E, Kang M, Yang DK, Kim JM, et al. PICOT inhibits cardiac hypertrophy and enhances ventricular function and cardiomyocyte contractility. Circulation research. 2006;99:307–14. doi: 10.1161/01.RES.0000234780.06115.2c. [DOI] [PubMed] [Google Scholar]
- Lal H, Verma SK, Smith M, Guleria RS, Lu G, Foster DM, et al. Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta1-integrin and focal adhesion kinase. Journal of molecular and cellular cardiology. 2007;43:137–47. doi: 10.1016/j.yjmcc.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Gong H, Ge J, Jiang G, Zhou N, Li L, et al. High density lipoprotein downregulates angiotensin II type 1 receptor and inhibits angiotensin II-induced cardiac hypertrophy. Biochemical and biophysical research communications. 2011;404:28–33. doi: 10.1016/j.bbrc.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Liu J, Shen Q, Wu Y. Simvastatin prevents cardiac hypertrophy in vitro and in vivo via JAK/STAT pathway. Life sciences. 2008;82:991–6. doi: 10.1016/j.lfs.2008.02.012. [DOI] [PubMed] [Google Scholar]
- McCain ML, Parker KK. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Archiv : European journal of physiology. 2011;462:89–104. doi: 10.1007/s00424-011-0951-4. [DOI] [PubMed] [Google Scholar]
- Methawasin M, Hutchinson KR, Lee EJ, Smith JE, Saripalli C, Hidalgo CG, et al. Experimentally increasing titin compliance in a novel mouse model attenuates the frank-starling mechanism but has a beneficial effect on diastole. Circulation. (3rd) 2014;129:1924–36. doi: 10.1161/CIRCULATIONAHA.113.005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedachi T, Fujita H, Kanzaki M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. American journal of physiology Endocrinology and metabolism. 2008;295:E1191–204. doi: 10.1152/ajpendo.90280.2008. [DOI] [PubMed] [Google Scholar]
- Nikolić N, Bakke SS, Kase ET, Rudberg I, Flo Halle I, Rustan AC, et al. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PloS one. 2012;7:e33203–e. doi: 10.1371/journal.pone.0033203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrotty DM, Klinger RY, Badie N, Hinds S, Kardashian A, Bursac N. Structural coupling of cardiomyocytes and noncardiomyocytes: quantitative comparisons using a novel micropatterned cell pair assay. American journal of physiology Heart and circulatory physiology. 2008;295:H390–400. doi: 10.1152/ajpheart.91531.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel DR, Adachi T, Ido Y, Heibeck T, Jiang B, Lee Y, et al. Strain-stimulated hypertrophy in cardiac myocytes is mediated by reactive oxygen species-dependent Ras S-glutathiolation. Journal of molecular and cellular cardiology. 2006;41:613–22. doi: 10.1016/j.yjmcc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. The Journal of cell biology. 2000;149:731–40. doi: 10.1083/jcb.149.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbaud S, Sanchez H, Garnier A, Fortin D, Bigard X, Veksler V, et al. Stimulus specific changes of energy metabolism in hypertrophied heart. J Mol Cell Cardiol. 2009a;46:952–9. doi: 10.1016/j.yjmcc.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Rimbaud S, Sanchez H, Garnier a, Fortin D, Bigard X, Veksler V, et al. Stimulus specific changes of energy metabolism in hypertrophied heart. Journal of Molecular and Cellular Cardiology. 2009b;46:952–9. doi: 10.1016/j.yjmcc.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Russell B, Curtis MW, Koshman YE, Samarel AM. Mechanical stress-induced sarcomere assembly for cardiac muscle growth in length and width. Journal of Molecular and Cellular Cardiology. 2010;48:817–23. doi: 10.1016/j.yjmcc.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling J, Kelly DP. The PGC-1 cascade as a therapeutic target for heart failure. Journal of molecular and cellular cardiology. 2011;51:578–83. doi: 10.1016/j.yjmcc.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt AJT, Rosen MR, Gaudette GR, Cohen IS. Repairing damaged myocardium: evaluating cells used for cardiac regeneration. Current treatment options in cardiovascular medicine. 2008;10:59–72. doi: 10.1007/s11936-008-0007-z. [DOI] [PubMed] [Google Scholar]
- Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell metabolism. 2008;7:33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Sheng L, Ye P, Liu Y-X, Han C-G, Zhang Z-Y. Peroxisome proliferator-activated receptor beta/delta activation improves angiotensin II-induced cardiac hypertrophy in vitro. Clinical and experimental hypertension (New York, NY : 1993) 2008;30:109–19. doi: 10.1080/10641960801945840. [DOI] [PubMed] [Google Scholar]
- Stewart LQ, Roper JA, Young WS, O'Carroll AM, Lolait SJ. Pituitary-adrenal response to acute and repeated mild restraint, forced swim and change in environment stress in arginine vasopressin receptor 1b knockout mice. Journal of neuroendocrinology. (3rd) 2008;20:597–605. doi: 10.1111/j.1365-2826.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen J-F, Newman M, et al. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. Journal of molecular and cellular cardiology. 2007;42:1137–41. doi: 10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibonnier M, Auzan C, Madhun Z, Wilkins P, Berti-Mattera L, Clauser E. Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J Biol Chem. 1994;269:3304–10. [PubMed] [Google Scholar]
- Tian M, Asp ML, Nishijima Y, Belury Ma. Evidence for cardiac atrophic remodeling in cancer-induced cachexia in mice. International journal of oncology. 2011;39:1321–6. doi: 10.3892/ijo.2011.1150. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wisloff U, Kemi OJ. Animal Models in the Study of Exercise-Induced Cardiac Hypertrophy. 2010;8408:633–44. doi: 10.33549/physiolres.931928. [DOI] [PubMed] [Google Scholar]
- Yasuda N, Miura S-i, Akazawa H, Tanaka T, Qin Y, Kiya Y, et al. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO reports. 2008;9:179–86. doi: 10.1038/sj.embor.7401157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Li L, Wu J, Gong H, Niu Y, Sun A, et al. Mechanical stress-evoked but angiotensin II-independent activation of angiotensin II type 1 receptor induces cardiac hypertrophy through calcineurin pathway. Biochemical and biophysical research communications. 2010;397:263–9. doi: 10.1016/j.bbrc.2010.05.097. [DOI] [PubMed] [Google Scholar]