Abstract
Aging is associated with progressive changes in learning and memory. A potential approach to attenuate age-related cognitive decline is cognitive training. In this study, adult male and female rats were given either repeated exposure to a T-maze, or no exposure to any maze, and then tested on a final battery of cognitive tasks. Two groups of each sex were tested from 6-18 months old on the same T-maze; one group received a version testing spatial reference memory, and the other group received only the procedural testing components with minimal cognitive demand. Groups three and four of each sex had no maze exposure until the final battery, and were comprised of aged or young rats. The final maze battery included the practiced T-maze plus two novel tasks, one with a similar, and one with a different, memory type to the practice task. The fifth group of each sex was not maze tested, serving as an aged control for the effects of maze testing on neurotrophin protein levels in cognitive brain regions. Results showed that adult intermittent cognitive training enhanced performance on the practice task when aged in both sexes, that cognitive training benefits transferred to novel tasks only in females, and that cognitive demand was necessary for these effects since rats receiving only the procedural testing components showed no improvement on the final maze battery. Further, for both sexes, rats that showed faster learning when young demonstrated better memory when aged. Age-related increases in neurotrophin concentrations in several brain regions were revealed, which was related to performance on the training task only in females. This longitudinal study supports the tenet that cognitive training can help one remember later in life, with broader enhancements and associations with neurotrophins in females.
Keywords: Cognitive training, Age-related memory decline, Aging, Learning, Memory, Spatial, Enrichment, Brain training/neurobics
1. Introduction
Life expectancy is continuing to increase in tandem with the baby-boom generation growing older. Projections to the year 2050 show 19 million Americans will be living into their 80s (Shrestha, 2005, Vincent and Velkoff, 2010). As age is the number one risk factor for developing Alzheimer's disease (AD; Lindsay, et al., 2002), this population faces a greater burden of both normal age-associated and pathological cognitive decline. Currently, there exist few treatments for AD, with only two classes of FDA approved drugs which possess short-term efficacy and do not prevent or halt the progression of AD; of note, these drugs are not prescribed for age-associated cognitive decline (Chiang and Koo, 2014, Petersen, et al., 2001). Remaining cognitively active may help protect against age-related cognitive decline and development of AD. In fact, that a non-pharmacological approach might protect against cognitive decline due to normal aging or neurodegenerative disease provides potentially new avenues for treatment.
Accumulating evidence supports the tenet that cognitive activity is related to cognitive prowess as aging ensues. For example, people 60 – 85 years old were able to improve their ability to multitask by participating in a video game activity for one month (Anguera, et al., 2013). Interestingly, these aged participants outperformed 20 year olds, and enhancements persisted for at least six months while transferring/benefiting other cognitive domains (Anguera, et al., 2013). Other forms of cognitive training also show benefit. Education is related to decreased risk of developing dementia and some symptoms of AD (Bowirrat, et al., 2002, De Ronchi, et al., 1998, Fritsch, et al., 2002, Gatz, et al., 2001, Letenneur, et al., 1999, Ott, et al., 1999), and more early- and mid- life cognitive activity is related to less β-amyloid deposition (Landau, et al., 2012). Moreover, college professors with 21-23 years of education maintained cognitive performance as they aged, with professors in their sixties performing as well as professors in their thirties (Shimamura, et al., 1995). Lastly, better cognitive ability as a child, as measured on a global scale of cognitive functioning, was associated with a decreased risk of developing late-onset dementia (Whalley, et al., 2000), and cognitive stimulation therapy is beneficial to people diagnosed with AD (reviewed in Ballard, et al., 2011). Collectively, there is evidence that multiple forms of cognitive activity attenuates age- and AD- related cognitive decline.
The research conducted in humans is largely corroborated by studies using animal models. For example, short-term working memory training in young adult mice enhanced selective attention and performance on a battery of tests (Light, et al., 2010). Furthermore, long-term cognitive training in rodents benefitted later performance on tasks assessing working and reference memory, as well as selective attention (Bierley, et al., 1986, Markowska and Savonenko, 2002, Matzel, et al., 2011, Vicens, et al., 2003); these effects do not appear to depend on visual acuity or swimming ability (Markowska and Savonenko, 2002), but do transfer to novel/untrained tasks and novel cognitive domains (Light, et al., 2010, Matzel, et al., 2011). The majority of this research has evaluated males; in fact, only one rodent study has evaluated females, and there was no male comparison group (Ando and Ohashi, 1991). Hence, it is unknown whether sex differences exist in response to cognitive training.
The neurobiological mechanism whereby cognitive activity might attenuate age-related cognitive decline is largely unknown. One potential mechanism is the neurotrophic system, which has been found to change with age (Mufson, et al., 1995). Neurotrophins, such as nerve growth factor (NGF), impact cholinergic neuron survival and maintenance (Granholm, 2000, Levi-Montalcini, 1987, Woolf, 1991), and numerous studies have demonstrated that age-related memory deficiency is coupled with alterations in the cholinergic system (e.g., humans: Drachman and Leavitt, 1974, rats: Eckerman, et al., 1980, monkeys: Rupniak, et al., 1990). Furthermore, NGF protein concentrations have been correlated with cognitive maze scores in aged rats (Bimonte-Nelson, et al., 2003a), and NGF administration has been shown to improve learning and memory deficits in aged rats (Backman, et al., 1996, Fischer, et al., 1987, Gustilo, et al., 1999, Markowska, et al., 1994, Scali, et al., 1994). Taken together, it is plausible that long-term cognitive training impacts NGF concentrations, which in turn could translate to an attenuation of age-related memory changes.
In the current study, we aimed to determine whether long-term cognitive training attenuates age-related cognitive decline in male and female rats, and, if so, what parameters guide these effects. Therefore, for male and female rats, this study was designed to evaluate: 1) whether the effects of cognitive training were due to cognitive demand and/or the procedural components of testing, and 2) whether effects were limited to the trained cognitive domain, or if they transferred to an untrained cognitive domain. Two recent studies found that cognitive demand is indeed necessary for the effects of cognitive training to be realized, and that benefits can transfer to novel tasks and novel cognitive domains (Light, et al., 2010, Matzel, et al., 2011); however, both studies evaluated only male mice. Furthermore, given that the human literature suggesting that cognitive ability (Whalley, et al., 2000), or cognitive reserve (Snowdon, 2003), impacts cognitive status when aged, we also assessed whether performance during the first cognitive training session when young related to later performance when aged. After testing on the final maze battery, NGF concentrations were assayed in several cognitive brain regions. This allowed us to evaluate whether NGF concentrations were altered by cognitive training and/or related to any cognitive-training induced learning and memory enhancements. We hypothesized that cognitive training would alter the trajectory of age-related cognitive decline and neurotrophic systems in a sex-specific manner. Indeed, given long-standing research suggesting that females are “buffered” against insult and show more plasticity in comparison to males (for review see Fitch and Bimonte, 2002, Juraska, 1986), we predicted a priori that females would be most benefitted by cognitive training.
2. Methods
2.1. Subjects
Six-month-old Fisher-344 rats (46 female, 50 male) were obtained from the National Institute on Aging (NIA) colony at Harlan Laboratories, and were pair housed in the Arizona State University (ASU) rodent facility in the department of Psychology. Rats had exposure to food and water ad libitum and were maintained on a 12-h light/dark cycle at 23°C. Procedures were approved by ASU's Institutional Care and Use Committee (IACUC), adhered to the Guide for the Care and Use of Laboratory Rats (National Research Council; 2011), and National Institutes of Health (NIH) standards. Following Markowska and Savonenko (2002), initiation of the cognitive and procedural component training schedule began at 6 months of age. Rats received one training session every 3 months; training continued for 12 months, with rats receiving their last training session at 18 months of age. Care was taken to ensure that the T-maze, and all objects and spatial cues in the training maze room, remained unchanged for the entire 12-month training and final maze battery testing periods. After behavioral testing, all rats were euthanized, and the rapid collection of several brain regions ensued immediately. At this experimental time point, the aged groups were 23 months of age, and the Young-Naïve group was 8 months of age. Later, NGF protein concentrations from the collected brain regions were assayed.
To meet the goals of the experiment, we used four groups of rats that were 6 months old at the beginning of the study and 21 months old at the beginning of the final maze battery, and one group of young rats that were 6 months old at the beginning of the final maze battery. This resulted in a total of five groups: 1) the aged cognitive training group which assessed the effects of reference memory training from 6 to 18 months of age (Aged Cognitive Training), 2) the aged procedural component group which addressed the effects of the procedural components of maze testing (Aged Swim Only), 3) the aged naïve group which had no maze exposure prior to the final maze battery (Aged Naïve), 4) the aged never-tested group which assessed the effects of age on NGF protein concentrations in rats that had no maze exposure (Aged Never Tested), and, 5) the young 6-month-old naïve group, which we included to compare aged groups to a group with “optimal” learning and memory ability (Young).
Treatment Groups
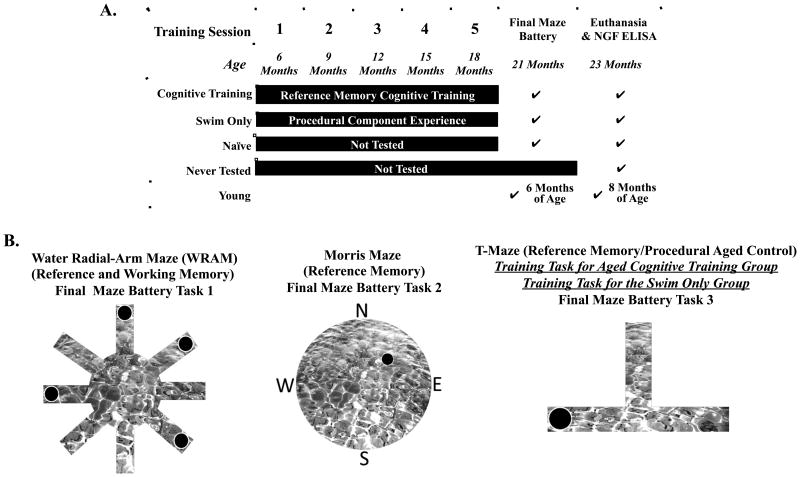
Figure 1A displays each treatment group and their age, concordant with a timeline of the experimental procedures. The Aged Cognitive Training group was composed of 19 rats (8 female, 11 male). From 6 to 18 months of age, this group was tested every 3 months for 5 days, 6 trials per day, on the reference memory T-maze using procedures previously published (Denenberg, et al., 1991, Denenberg, et al., 1990, Gibbs, et al., 2004). The black Plexiglas® maze (each arm was 38.1cm × 12.7cm) was filled with water made opaque with black non-toxic paint, and had a hidden escape platform with a wire mesh top at the end of one of the two non-start arms. Each rat had an assigned exit location (East or West) for the entire duration of training through the final maze battery. Drop off locations varied between north and south arms to minimize the possibility of rats using a motoric/olfactory strategy rather than a spatial strategy. After a rat located the platform, it remained there for 5 sec; the rat was then removed and placed in its heated cage for 30 sec until the next trial. Between trials the maze was cleaned and water disrupted to minimize the possibility of rats using an olfactory strategy. The dependent variable was the number of correct arm entries, quantified as the number of times a rat found the hidden escape platform on the first choice of each trial within a day.
Figure 1.
(A) Timeline and design of the experiment. (B) A diagram of the mazes used in this study. The spatial working and reference memory WRAM was the first novel task in a novel environment that behaviorally tested groups were exposed to during final testing battery. The spatial reference memory Morris maze was the second novel task in a novel environment used during final maze battery. The T-maze was used as the reference memory training task, and modified for use as the procedural components task, and it was tested last during final maze battery.
The Aged Swim Only group consisted of 21 rats (10 female, 11 male). From 6 to 18 months of age, this group experienced conditions identical to the Aged Cognitive Training group, with the exception that the T-maze was modified to minimize cognitive demand. Exit arms were assigned randomly for each trial pair. Cognitive demand was minimized by keeping the non-exit, non-platformed, arm blocked; hence, the escape arm was the only arm left open. This resulted in this group receiving similar experience as the Aged Cognitive Training group regarding being removed from the colony room, and experiencing handling and maze testing. Drop off locations varied between north and south arms to maximize swimming similarities to the Aged Cognitive Training group. Inclusion of the Aged Swim Only group addressed whether the procedural components of maze testing throughout the training period influenced age-related cognitive change.
The Aged Naïve group consisted of 20 rats (10 female, 10 male) which remained in their colony room cages from 6 to 21 months of age (i.e., the entire training period), and then were tested on the final maze battery. The Aged Naïve group allowed us to reference the normally aged laboratory rat that did not receive cognitive training or prior behavior testing.
The Aged Never Tested group was composed of 18 rats (9 female, 9 male) that remained in their colony room cages from 6 to 23 months of age (i.e., the entire study period) and were never behaviorally test. In fact, these rats were not handled before euthanasia aside from normal caretaking procedures (e.g., cage changes). Aged Never Tested rats were euthanized along with the other groups of rats after the final maze battery. We used this group to evaluate NGF concentrations in the cognitive brain regions of rats that were not exposed to behavioral testing.
The Young group was comprised of 18 (9 female, 9 male) adult rats. This group was tested only during the final maze battery, so that we could compare the aged groups to the cognitively-intact, non-experimentally manipulated, young rat. These rats were 6 months of age when tested on the final maze battery. This age was chosen for the Young group because this is the age when the cognitive training period started for the Swim Only and Cognitive Training groups.
2.2 Vaginal and control anal swabs
After the completion of all training sessions, just prior to the initiation of the final maze battery, all females received vaginal smears once a day in the morning for several consecutive days following previously published methods (Acosta, et al., 2009b, Talboom, et al., 2010). Vaginal cytology was classified as proestrus, estrous, metestrus, or diestrus (Goldman, et al., 2007). All aged females were categorized as being in an estropause state. Nearly all of the aged females remained in anestrus; the majority of the aged females consistently displayed a constant diestrus smear, with overall few cells that were primarily leukocytes, consistent with anestrus and relatively stable circulating concentrations of ovarian hormones. Very few aged females displayed transitioning diestrus- and proestrus- like smears. The transitioning proestrus-like smear consisted of epithelial cells, intermediate cells, and noticeable mucus. All young females displayed normal four day cycles, indicative of normal reproductive capacity and fluctuating concentrations of circulating ovarian hormones. To control for the handling and procedure of vaginal swabs, all males received a gentle anal probe with a swab using a nearly identical procedure to the one used to perform vaginal swabs in the females.
2.3. Mazes used during the final maze battery
We began the final maze battery when the aged groups (Aged Cognitive Training and Aged Control) were 21 months of age. The final maze battery included mazes assessing both working and reference memory. We chose these mazes to determine whether cognitive training on one maze (the T-maze) benefited performance only on the same maze (the T-maze), or whether effects transferred to untrained mazes (the water radial-arm maze [WRAM] and Morris maze). Further, because the final maze battery included mazes assessing both working and reference memory, we could also determine whether memory transfer effects to novel mazes occurred only within the tested memory domain (reference memory), or whether they transferred to an untrained memory domain (working memory) as well. The final maze battery, on which all groups except the Aged Never Tested group were evaluated, included the following mazes in the order of final battery testing: the win-shift working memory and reference memory WRAM, the reference memory Morris maze, and the reference memory T-maze. The WRAM and Morris maze were novel tasks, assessing performance in novel environments/testing rooms. The T-maze was a familiar task in a familiar environment/testing room to the Aged Cognitive Training and Aged Swim Only groups, except cognitive demand was now required of the Aged Swim Only group. Each maze was located in a separate, distinct room that had salient, visual, extra-maze cues that remained constant throughout the testing period.
2.3.1. WRAM
The win-shift WRAM is an 8-arm maze (Figure 1B) which was constructed of black Plexiglas® and filled with room temperature water made opaque using black non-toxic paint (e.g., Bimonte and Denenberg, 1999, Bimonte and Denenberg, 2000, Bimonte, et al., 2000). Hidden escape platforms (1 cm below the water) were placed in the ends of 4 of the 8 arms. Each subject had 4 platform locations that remained fixed throughout testing. A subject was released from the start arm and had 3 min to locate a platform; if it did not find a platform during the 3 min, it was gently led, using a dark rod, to the closest one. Once on a platform, the rat remained on it for 15 sec, and was then returned to its heated cage for a 30 sec inter-trial-interval (ITI). During the ITI, the just located platform was removed from the maze. The rat was then placed again into the start arm and allowed to locate another platform. For each rat, a daily session consisted of 4 trials, with the number of platformed arms reduced by 1 on each subsequent trial. Thus, the working memory load was increasingly taxed as trials progressed. Each rat was given 1 session a day for 12 consecutive days. Quantification and blocking procedures were based upon previous studies using the WRAM (Bimonte-Nelson, et al., 2003a, Bimonte, et al., 2002, Bimonte, et al., 2003). Errors were quantified using orthogonal measures of working and reference memory (Jarrard, et al., 1984), as done in previous WRAM studies (see Bimonte-Nelson, et al., 2003a, Bimonte, et al., 2002, Bimonte, et al., 2000, Hyde, et al., 2000). Working memory correct (WMC) errors were the number of first and repeat entries into any arm from which a platform had been removed during that day. Reference memory (RM) errors were the number of first entries into any arm that never contained a platform within a day. Working memory incorrect (WMI) errors were the number of repeat entries into an arm that never contained a platform within a day (i.e., repeat entries into a reference memory arm within a day). Number of WMC, WMI, and RM errors served as the dependent variables for statistical analysis, and data were blocked into 4-day blocks (Bimonte-Nelson, et al., 2003a, Bimonte, et al., 2002, Bimonte, et al., 2003).
2.3.2. Morris maze
The Morris maze (see Fig. 1B for a diagram of the maze, Morris, et al., 1982) evaluated spatial reference memory, and consisted of a round tub (188 cm in diameter) filled with water made opaque with black non-toxic paint. The rat was placed in the maze from any of four locations (North, South, East, or West) and had 60 sec to locate a submerged hidden escape platform with a wire mesh top which remained in a fixed location (the target Northeast quadrant, NE) throughout testing. After 15 sec on the platform, the rat was placed into its heated cage until the next trial; the inter-trial-interval (ITI) was 5-8 min. For each rat, the testing session consisted of 4 trials/day for 5 days. A video camera recorded each rat, and a tracking system (EthoVision 3.1, Noldus Information Technology, Wageningen, Netherlands) analyzed each rat's path. The dependent measure was swim distance (cm), with less swim distance interpreted as better performance. To assess platform localization, a probe trial was given on an additional trial (trial 5) on the last day of testing, whereby the escape platform was removed from the maze. The dependent measures for the probe trial were percentage of total swim distance (cm) in the target NE quadrant as compared to the opposite Southwest (SW) quadrant.
2.3.3. T-maze
The procedure for the T-maze during the final maze battery was identical to that given to the Aged Cognitive Training group during the training period, as described above. For the rats with T-maze experience from the training sessions, the platform location remained constant (in the same arm of the maze, and same place in space) during cognitive training and the final maze battery. The dependent variable was the number of correct arm entries made within a daily session.
2.4. Brain dissection and NGF analysis
At sacrifice, rats were anesthetized with isoflurane (Vetone, Meridian, Indiana) and euthanized according to NIH guidelines and American Veterinary Medical Association (AVMA) guidelines on euthanasia (2007), after which the brains were rapidly dissected. Following a stereotaxic atlas (Paxinos and Watson, 2005) right hemisphere frontal cortex, parietal cortex, temporal cortex, striatum, entorhinal cortex, and ventral hippocampus (CA1/2) were rapidly dissected, as done previously (Bimonte-Nelson, et al., 2003a). Dissected tissues were immediately placed in weighed microcentrifuge tubes, frozen on dry ice, and stored at -70 °C until tissue lysates were made. NGF protein concentrations were assessed via enzyme-linked immunosorbent assay (ELISA) using commercially available kits from Promega (Madison, Wisconsin). NGF assay procedures were conducted as previously described (Engler-Chiurazzi, et al., 2009). Briefly, tissue lysates were made, processed, and read in flat-bottom 96 well plates according to the kit's instructions. The range of NGF detection was between 4.7-300 pg/ml and 7.8-500 pg/ml. For each assay kit, cross-reactivity with other trophic proteins was < 2-3%. The concentration of NGF (pg/mg of tissue) present in the brain region of interest was the dependent variable used for statistical analysis.
2.5. Statistical methods
The current study was designed to determine whether cognitive training enhanced performance in aged animals, whether there was an age effect on each maze, and whether the cognitive training or age effects differed by sex. Therefore, we aimed to compare the Young group to the Aged Control group to determine age effects, and the Aged Control group to the Aged Cognitive Training group to determine cognitive training effects in the aged animals. As a result, comparisons were planned a priori and Type I error correction was not necessary (Keppel and Wickens, 2004). Data were analyzed using a mixed model ANOVA with either Age or Treatment, and Sex, as the between variables, and repeated measures variables as appropriate for the specific test. This statistical routine is common (e.g. Braden et al., 2011), and was necessary so that we could evaluate potentially complex higher order interactions with Days and/or Trials for behavior assessments. To evaluate potential relations between maze performance when young and NGF concentrations when aged, one predictor regression analysis including Pearson's correlation coefficient (r) was run within the Cognitive Training group. All ANOVAs, regressions, and correlations were performed using StatView (for Windows version 5.0.1; SAS Institute Inc.; Cary, NC); the data were then graphed using Prism (version 6.04, GraphPad Software, La Jolla, CA).
We employed linear growth modeling in the current study as well. Paralleling research conducted in humans (e.g., Whalley, et al., 2000), we wished to evaluate whether cognitive ability when young related to cognitive ability when aged. We estimated a linear growth model from the number of correct arm entries made by the young Cognitive Training group across their first session on the cognitive training T-maze. The model was predicted as a function of the elapsed number of days from the initial cognitive training session conducted when the rats were 6 months of age (Singer and Willett, 2003). Specifically, we used growth modeling to determine if initial cognitive ability/performance (intercept) or rate of learning (slope) differed between males and females, and if ability or rate of learning when young was related to the novel task cognitive performance when aged (described in more detail below). We used Mplus (version 5.21; Muthén & Muthén; Los Angeles, California) to estimate the linear growth model.
3. Results
At the conclusion of the study, statistical analyses were performed to evaluate potential differences between the Aged Swim Only and Aged Naïve groups for behavior, and the Aged Swim Only, Aged Naïve, and Aged Never Tested groups for the NGF analysis; no significant differences would allow simplification of the analyses since these groups could be combined into one control group for each respective analysis. There were no significant differences between the two groups for behavior for any dependent measure showing cognitive training differences, nor were there significant differences between the three groups for the NGF analysis.
3.1. WRAM
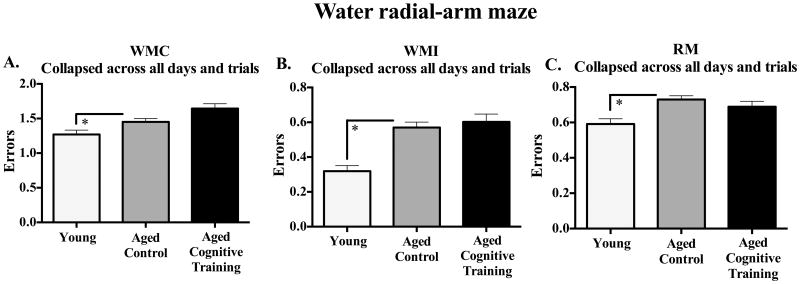
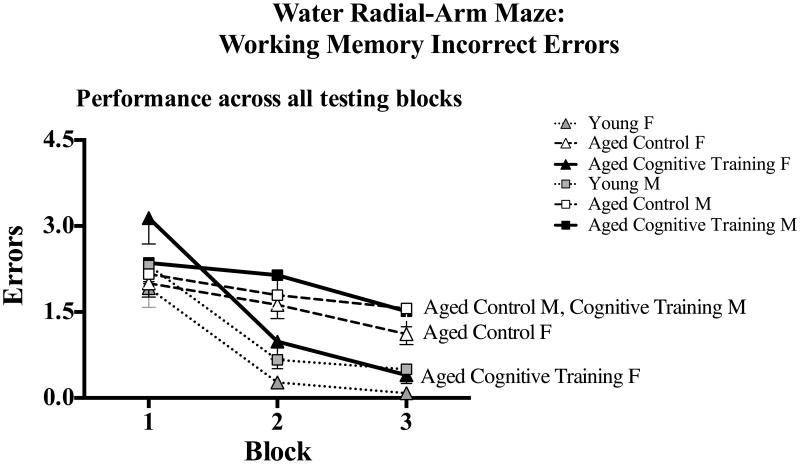
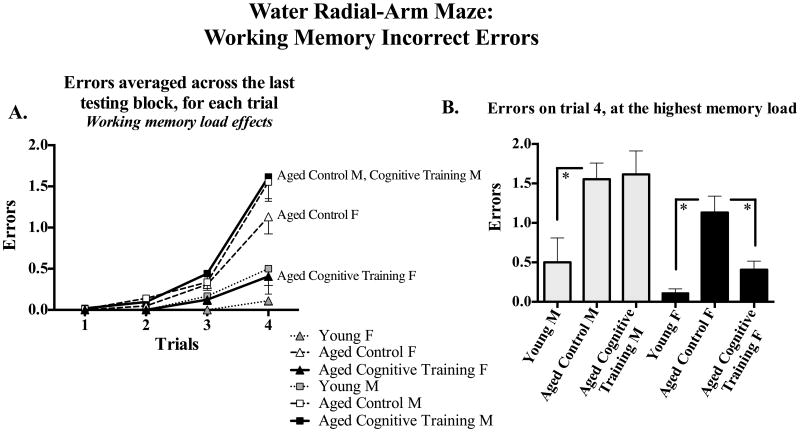
Figure 2 shows the mean ±SE error scores for each group, collapsed across all blocks and trials. Young rats outperformed Aged Control rats for WMC [F(1,56) = 4.40, p < 0.05; Figure 2A], WMI [F(1,56) = 16.77, p < 0.0001; Figure 2B], and RM [F(1,56) = 23.28, p < 0.0001; Figure 2C], with no Age × Sex interaction. For the Aged Control and Aged Cognitive Training group analysis, there were no main effects or interactions with Sex for WMC or RM. Effects were specific to WMI. Figure 3 shows the mean ±SE error scores for each block for each group for WMI. For WMI, there was no main effect of Treatment, and there was a significant Treatment × Block [F(2,116) = 3.26, p < 0.05] and Treatment x Block x Trial interaction [F(6,348) = 2.13, p < 0.05]. Further WMI analysis of the last block of testing (for the asymptotic phase of performance, as done routinely to test memory after learning has occurred; e.g., Acosta, et al., 2009a, Bimonte-Nelson, et al., 2003b, Bimonte-Nelson, et al., 2004, Bimonte and Denenberg, 1999, Digby, et al., 2012), revealed a significant Sex x Trial interaction [F(3,183) = 8.51, p < 0.0001] and a marginal Treatment × Sex × Trial interaction [F(3,183) = 2.14, p = 0.09], as shown in Figure 4A. Since the working memory load increases as trials increase, an interaction with trial could indicate a differential ability to handle an increasing working memory load. Thus, for WMI we tested the trial at the highest working load, trial 4, as done previously using this task (Acosta, et al., 2009a, Braden, et al., 2010, Digby, et al., 2012). As shown in Figure 4B, for WMI Aged Cognitive Training females made fewer errors than Aged Control females when working memory load was the highest [F(1,27) = 4.85, p < 0.05], an effect not found in males, as Aged Cognitive Training males did not differ from Control males [F(1,34) = 0.03, p = 0.86].
Figure 2.
Mean±SE number of: (A) WMC, (B) WMI, and (C) RM errors, committed on the WRAM collapsed across all blocks and trials. For each error type, Young animals outperformed the Aged Control animals. * p < 0.05.
Figure 3.
Mean±SE number of WMI errors committed on the WRAM across the three testing blocks (composed of 4 days each), collapsed across all trials, for each group.
Figure 4.
(A) Mean±SE number of WMI errors committed across trials during Block 3, the last block of testing. Working memory load increases as trials progress. (B) Mean±SE number of WMI errors made at the trial with the greatest working memory demand, trial 4. Aged Cognitive Training females made fewer errors than Aged Control females when working memory load was the highest, whereas Aged Cognitive Training males did not show an enhanced ability to manage this highest working memory load relative to Aged Control males. * p < 0.05.
3.2. Morris maze
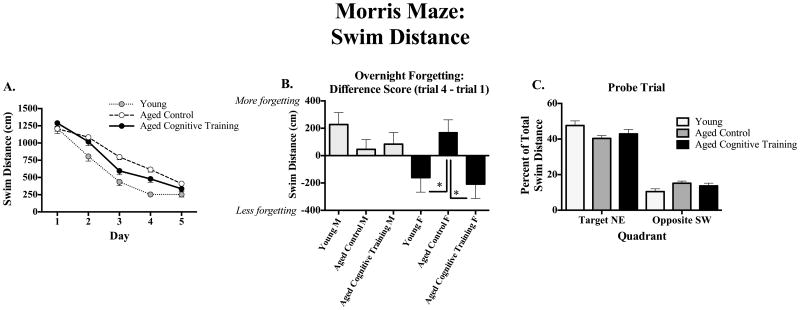
Figure 5A shows the mean ±SE swim distance scores across days for each group. Young animals exhibited better performance than Aged Controls, as Young animals had lower swim distance scores than Aged Controls [F(1,56) = 18.01, p < 0.0001]; there were no significant Age × Sex interactions. For the analysis of the Aged Control and Aged Cognitive Training groups, there was no main effect of Treatment, and there was a significant Treatment × Day interaction [F(4,232) = 2.99, p < 0.05]; Aged Cognitive Training rats showed better performance starting on day 3. Since these analyses revealed that the Cognitive Training group demonstrated better performance starting on day 3, we evaluated overnight forgetting of the platform location during the latter block of testing (overnight forgetting for days 2 to 3, days 3 to 4, and days 4 to 5), by computing and testing the difference in swim distance between the first trial of the next day after the overnight interval, and the last trial of the previous day (trial 1 distance – trial 4 distance). Overnight forgetting is shown for all groups in Figure 5B. For this assessment of overnight forgetting, there was a significant Cognitive Training × Sex interaction [F(1,59) = 4.67, p < 0.05]; Aged Cognitive Training females outperformed Aged Control females [F(1,27) = 5.86, p < 0.05]. This effect was not found in their male counterparts, as Aged Cognitive Training males did not differ from Aged Control males [F(1,32) = 0.11, p = 0.74]. Female Aged Control rats and had more overnight forgetting than female Young rats [female Aged Control vs. female Young: F(1,28) = 4.08, p ≤ 0.05]; this effect was not seen in males [male Aged Control vs. male Young: F(1,29) = 1.90, p = 0.18].
Figure 5.
(A) Learning: Mean±SE swim distance (cm) across days on the Morris maze, collapsed across all trials. Young animals performed better than Aged Control and Aged Cognitive Training groups. (B) Overnight forgetting: Mean±SE difference in swim distance (cm) from trial 1 to trial 4 (trial 1 – trial 4), collapsed across days 2-5. Aged Cognitive Training females outperformed Aged Control females, an effect not found in males. (C) Probe trial: Mean±SE percent of total swim distance (cm) in the target NE vs. opposite SW quadrants during the probe trial. Young animals spent a greater percent of their total swim distance in the Target quadrant and less percent of total swim distance in the Opposite quadrant, than Aged Control animals, regardless of cognitive training or sex. Thus, all groups were able to equally localize the platform quadrant by the end of testing. * p < 0.05.
Figure 5C shows the mean ±SE probe trial data for each group. For the probe trial, for the Young and Aged Control comparison, all animals spent a greater percent distance in the Target versus Opposite quadrant [main effect of Quadrant: F(1,55) = 182.01, p < 0.0001], and there was a significant Treatment × Quadrant interaction (F[1,55] = 6.58, p < 0.05). Further analyses to understand this interaction showed that Young animals swam somewhat more in the Target quadrant [percent of total swim distance in NE quadrant: Aged Control vs. Young F(1,57) = 3.12, p = 0.08], but swam less in the Opposite quadrant [percent of total swim distance in SW quadrant: Aged Control vs. Young F(1,55) = 4.99, p < 0.05] when compared to Aged Control animals, and there were no Sex × Quadrant, nor Sex × Age × Quadrant, interactions. For the Aged Control vs. Aged Cognitive Training comparison, there was a main effect of Quadrant [F(1,57) = 147.75, p < 0.0001], with both Aged Control and Aged Cognitive Training groups spending a greater percent distance in the Target quadrant; there were no Sex × Quadrant, nor Sex × Cognitive Practice x Quadrant, interactions, indicating that all aged groups were able to equally localize the platform quadrant by the end of testing.
3.3. T-maze
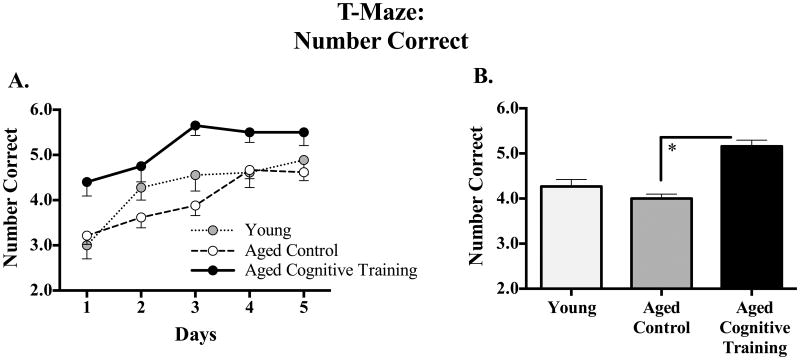
Figure 6A shows the mean ±SE number correct for each group for each day, and Figure 6B shows the mean ±SE number correct for each group collapsed across days. There was no effect of Age, nor an Age × Sex interaction, for the T-maze. The Aged Cognitive Training group outperformed the Aged Control group for the T-maze final battery assessment [Treatment main effect: F(1,58) = 23.73, p < 0.0001], an effect which did not interact with Sex.
Figure 6.
(A) Mean±SE number of correct arm choices made across all days of testing on the T-Maze, collapsed across all trials. (B) Mean ±SE number of correct arm choices made collapsed across all days and trials. Aged Cognitive Training animals performed better than that Aged Control animals. * p < 0.05.
3.4. NGF protein concentrations and correlations with behavior
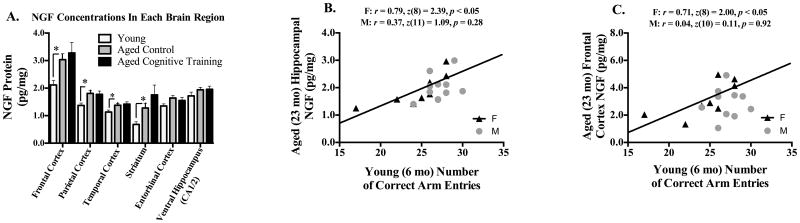
Figure 7A shows the mean ±SE NGF protein values for each brain region, for each group. Aged Control animals had higher concentrations of NGF in the frontal cortex [F(1,71) = 6.97, p < 0.05], parietal cortex [F(1,71) = 7.52, p < 0.05], temporal cortex [F(1,71) = 4.42, p < 0.05], and striatum [F(1,70) = 5.13, p < 0.05; of note, one sample was lost due to experimental error], as compared to Young animals; Age did not interaction with Sex for any brain region. NGF concentrations did not change with Cognitive Training (the Aged Cognitive Training group did not differ from the Aged Control group), nor did Cognitive Training interact with Sex for any brain region.
Figure 7.
(A) Mean±SE NGF protein concentration (pg/mg of tissue) for each brain region. The Young group had lower concentrations of NGF in the frontal cortex, parietal cortex, temporal cortex, and striatum. (B) Represented is the scatterplot of ventral hippocampal (CA1/2) concentrations of NGF (pg/mg tissue) in aged (23 month old) females (black filled triangles) and males (gray filled circles), and the number of correct arm choices made on the T-Maze during the first training session when young, 17 months earlier (at 6 months old). (C) Represented is the scatterplot of frontal cortex concentrations of NGF (pg/mg tissue) in aged (23 month old) females (black filled triangles) and males (gray filled circles), and the number of correct arm choices made on the T-Maze during the first training session when young, 17 months earlier (at 6 months old). Higher concentrations of NGF in the aged ventral hippocampus (CA1/2) and frontal cortex, significantly correlated with better initial spatial reference memory performance when young, in females only, as represented by the black solid line. F=females, M=males, * p <0.05.
We found a positive correlation between how well male and female rats performed on the initial exposure to the training task when young (6 months old) and their concentration of hippocampal NGF when aged at 21 months old [b = 0.10, r = 0.59, z(19) = 2.72, p < 0.05]. However, analyses of males and females alone revealed that this significant correlation was carried by the females [Females: b = 0.13, r = 0.79, z(8) = 2.39, p < 0.05; Males: b = 0.10, r = 0.37, z(11) = 1.09, p = 0.28; Figure 7B]. In fact, when females were evaluated separately, we found another positive correlation between how well females performed on the initial exposure to the training task when young, and their concentration of frontal cortex NGF when aged [b = 0.14, r = 0.71, z(8) = 2.00, p < 0.05, Figure 7C]. This identical correlation was not found to be significant when males were evaluated alone [b = 0.06, r = 0.04, z(10) = 0.11, p > 0.05].
3.5. Growth modeling
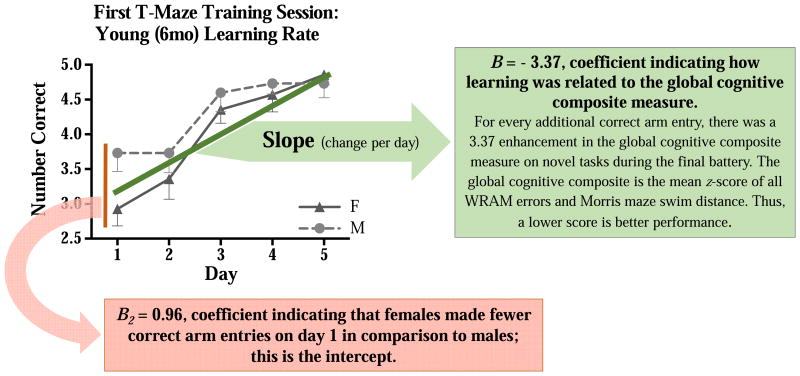
The linear growth model evaluating the first reference memory T-maze training session, when the Cognitive Training rats were young (6 months old), indicated that female rats had a steeper slope across days than males, suggesting that females learned the task at a faster rate compared to males [B3 = -0.27, p < 0.05, Figure 8]. Of note, females made fewer correct arm entries on day 1 in comparison to males [B2 = 0.96, p = .003; wherein B2 was the amount by which values of performance on day one differed between females and males], which would impact the slope of this line. Hence, females may have learned at a faster rate, and thereby reached the same level of performance seen in the males, by the end of the first practice session.
Figure 8.
Mean±SE number of correct arm choices across days on the T-Maze during the first maze session. Specifically, the graph displays the learning curve of male and female Aged Cognitive Training rats during their initial/first cognitive training session, when they were 6 months old. The linear growth model demonstrated that female rats had a steeper slope across days than males, suggesting that females learned the task at a faster rate [B3 = -0.27, p < 0.05]. Because females made fewer correct arm entries on day 1 in comparison to males [B2 = 0.96, p = .003, assessing the intercept], this impacted the slope of the line. A global cognitive composite measure was calculated for the novel tasks so that relationships between learning when young, and learning when old, could be evaluated. The number of WRAM total errors and swim distance on the Morris maze (all days and trials for both mazes), were converted to z-scores. These z-scores equally weighted the results from the two mazes, even though each maze used a different dependent variable metric (errors committed and swim distance, respectively). For both sexes, rats that learned the training task faster demonstrated enhanced performance when aged on the composite measure of the novel tasks tested during the final maze battery [B = - 3.37, p < 0.05]. This association between the learning rate when young and subsequent aged memory performance was strong according to Cohen's (1988) conventional norms in psychological science [r = 0.83, p < 0.05].
We created a global cognitive composite measure for the novel tasks so that we could evaluate relationships between learning when young, and learning when old. Specifically, the number of WRAM total errors (all three measures) and swim distance on the Morris maze, across all days and trials for both mazes, were converted to z-scores. This allowed us to create a single score representing overall performance on mazes novel to all tested groups during the final maze battery. The z-scores equally weighted the results from the two mazes even though each maze used a different dependent variable metric (i.e., errors committed and swim distance, respectively). For both sexes, rats that learned the training task faster demonstrated enhanced performance when aged on the composite measure of the novel tasks in the final maze battery [B = - 3.37, p ≤ 0.05, Figure 8]. This positive association between the learning rate of a rat when it was young and its subsequent aged memory performance was strong according to Cohen's (1988) conventional norms in psychological science [r = 0.83, p < 0.05].
4. Discussion
This longitudinal study supports the tenet that cognitive training can help one remember later in life. Here, we show that intermittent cognitive training throughout adulthood can attenuate age-related memory decline, but only when there is cognitive demand. The current study suggests that there are sex differences in this effect, with females showing benefits of cognitive training on novel tasks evaluated in the final battery, and males showing a lack of cognitive training-mediated benefits on novel tasks in the final battery. Hence, findings from this study indicate that females are able to transfer the training effects to untrained memory domains, and, at least within the parameters utilized here, males are not. Specifically, in the female rat, cognitive training on a reference memory maze task every three months from 6 to 18 months of age enhanced later cognitive performance when aged, at 21 months old, on tasks that were new to the animal. In females, on the final maze battery, a history of cognitive training from young adulthood to old age attenuated age-related working memory decline on a novel task (WRAM, as shown for the latter portion of testing for one of the two working memory measures examined on this task, WMI), protected against overnight forgetting on a novel reference memory task (Morris maze), and improved performance on the practiced reference memory training task (T-maze). In males, cognitive training-induced enhancements were only seen for the practiced reference memory training task (T-maze). It is noteworthy that the Treatment × Day interaction for the spatial reference memory Morris maze task initially indicated that cognitive training might have helped both sexes; however, noting the interactions with Sex and upon further analyses, it became clear that the cognitive training benefit was due to females showing enhanced performance for overnight retention for the latter three overnight intervals. Here, we also show that cognitive demand was necessary for cognitive training effects to be realized, since rats that received only the procedural testing components for training showed no improvement on the final maze battery. Moreover, male and female rats that had a faster rate of learning when young demonstrated better global performance on the novel tasks when aged. We also show here that NGF concentrations increased in several brain regions as aging ensued, and that NGF protein levels in the hippocampus and frontal cortex related to performance on the training task only in young females.
4.1. Sex interacts with the impact of adult lifelong cognitive training
The data presented here suggest that male and female rats differ in response to cognitive training administered across adulthood. For the parameters utilized in the current study, females benefited from cognitive training, and were the only sex to demonstrate enhanced performance, relative to controls, on novel tasks. That females benefit from cognitive training when aged is corroborated by one other study (Ando and Ohashi, 1991); however, in this study there was no male comparison group to evaluate sex differences. While females did learn the practice task faster, all rats reached and maintained near asymptotic performance by the end of their first cognitive training session, thereby limiting the possibility that the sex differences found during the final maze battery were due to differential learning of the cognitive training task. Numerous studies in males have demonstrated training-mediated enhancements of memory (e.g., Bierley, et al., 1986, Light, et al., 2010, Markowska and Savonenko, 2002, Matzel, et al., 2011, Vicens, et al., 2003); thus, it might initially appear surprising that we did not see more robust effects in males. However, the majority of these studies used the same or similar tasks for both the practice task and final assessment. This does correspond to our findings here, as we found that both males and females showed cognitive training-induced enhancements on the T-maze, which was used for both training and again testing on the final battery. Thus, it may be that both males and females show training-induced enhancements on the same task used for practice, but that females can transfer these benefits to a more global nature, showing benefits on other non-practiced tasks. Taken together with data suggesting females exhibit greater brain plasticity (for review see Fitch and Bimonte, 2002, Juraska, 1986), it may be that the training effects found previously in males might in part be due to “practice effects” and not true training-mediated enhancements to aged performance. It remains to be determined whether sex differences would still exist with cognitive training if the cognitive training sessions were initiated more frequently or with a different level of intensity. In addition, we would be remiss if we did not acknowledge that different estropause states may have impacted the effects of cognitive training. Indeed, estropause status has been found to influence cognition in aged rats (see Markowska, 1999, Warren and Juraska, 2000). In the current study, vaginal smears demonstrated that nearly all aged females were in an anestrus state, while the few found to be in a non-anestrus state were distributed throughout the various aged groups. Hence, while we cannot rule out possible estropause state-mediated alterations in the effect of cognitive training, the likelihood of its impact on our cognitive practice-induced sex difference findings is likely not largely accounting for the effects. However, of note, a systematic evaluation of interactions between state of estropause and efficacy of cognitive practice during aging is necessary to directly test this question, and could yield telling insight into the trajectory of sex differences as aging ensues, with and without cognitive practice.
4.2. Generalizing the impact of cognitive training: effects of long-term cognitive training transfer to untrained memory domains
Cognitive training protected aged female rats against the age-related decrements when handling an increasing working memory load on a novel working memory task, the WRAM. The WRAM is sensitive to rodent age-related working memory decline (Bimonte-Nelson, et al., 2003c, Bimonte, et al., 2002, Bimonte, et al., 2003), an effect we also demonstrate here. Reference memory cognitive training also attenuated age-related memory decline on the novel reference memory task, the Morris maze. This finding is corroborated by rodent experiments showing that long-term cognitive training enhances later performance on tasks assessing working and reference memory, as well as selective attention (Bierley, et al., 1986, Markowska and Savonenko, 2002, Matzel, et al., 2011). We also show here that reference memory cognitive training enhanced performance when aged on the training task, the reference memory T-Maze. Aged Cognitive Training animals performed better on the T-Maze cognitive training task during the final maze battery when compared to Aged Control animals, and Aged Naïve animals did not differ from Aged Swim Only animals that received only the procedural components of testing, indicating that cognitive training-induced enhancements are found even when control is imposed over the procedural components of testing. Of note, an age effect was not seen on the T-Maze during the final maze battery (i.e., the Aged Control vs. Young comparison). This lack of an age effect corroborates previous research wherein no age effects were found on a land T-Maze; only the type of solving strategy differed by age (Barnes, et al., 1980).
Since we found that cognitive training in females can protect against age-related decline, and that these effects were observed on mazes and environments novel to the group that had cognitive training, the effects could not simply be due to familiarity or prior experience with the specific task. The enhanced performance of the Cognitive Training group (both males and females) relative to the Control group on the T-maze during the final maze battery could be, in part, due to retention, and not relearning, of the escape platform location since their last cognitive training session. Scores were essentially asymptotic with little to no variation after the first cognitive training session; this level of performance persisted to the last training session at 18 months. Collectively, these data suggest that previous cognitive training enhances performance on the training task for both males and females.
4.3. Evidence that long-term adult cognitive training-induced enhancements require cognitive demand
Our data suggest that memory protection from cognitive training is due to the cognitive demand of the task, and not exposure to the procedural components of maze testing. During training, the Swim Only group had a single swim path without any choice points during the trials, thereby minimizing cognitive demand; this group “won” every time. This group, receiving only the procedural aspects of the task including being moved from the colony to the testing room, handling required of testing, swimming, and escaping on the platform, exhibited no protection from age-related memory decline and were virtually indistinguishable from aged controls that sat in their home cages until the final maze battery (the Aged Naïve rats). This finding is corroborated by recent studies where cognitive demand was necessary for enhancements of selective attention in young adult and aged male mice (Light, et al., 2010, Matzel, et al., 2011).
4.4. A faster rate of learning when young relates to superior global memory when old
The rate at which a male or female rat learned the cognitive training task during the first exposure at 6 months of age was related to cognitive performance when aged; indeed, a faster rate of learning when young corresponded to better memory on the novel mazes when aged. In the context of Donald Hebb's (1949) postulate describing the adaptation of neurons during learning and engram creation, it is tempting to speculate that learning when young strengthens brain networks so that enhanced learning and memory can occur when aged. Better learning when young might organize the brain in such a fashion that results in better learning later in life. Indeed, one might presume that such a tenet could underscore the idea that “cells that fire together, wire together” (Goodman and Shatz, 1993, Hebb, 1949), with prior learning providing greater cognitive reserve, in turn leading to better memory when challenged with a potentially cognitively-compromising situation, such as aging.
4.5. NGF protein concentrations were higher in aged rats, and concentrations in the frontal cortex and hippocampus when aged related to cognitive ability when young in females only
We evaluated NGF protein concentrations in the frontal cortex, parietal cortex, temporal cortex, striatum, entorhinal cortex, and ventral hippocampus (CA1/2), brain regions known to subserve cognitive and motor functions (Hasselmo, 2006, Woolf, 1991). Survival and maintenance of cholinergic neurons, which are critical for memory processes, are dependent upon neurotrophins including NGF (Bimonte-Nelson, et al., 2008, Bimonte-Nelson, et al., 2003a, Granholm, 2000, Levi-Montalcini, 1987, Siegel and Chauhan, 2000, Woolf, 1991). We observed here an age-related increase in NGF protein concentrations in the frontal cortex, parietal cortex, temporal cortex, and striatum, although cognitive training did not alter NGF concentrations in the brain regions assayed. The current NGF findings agree with our previous data showing that both male and female aged Fischer-344 rats exhibited higher NGF protein concentrations in the striatum and frontal cortex when compared to young counterparts (Bimonte-Nelson, et al., 2008, Bimonte-Nelson, et al., 2003a, Koh and Loy, 1988, Larkfors, et al., 1987).
In the current study, in females only, better performance on the cognitive training task when young was associated with higher concentrations of frontal cortex and hippocampal NGF protein concentrations when aged. These findings correspond to studies showing that NGF infusions into the aged rat brain can attenuate age-related memory deficits (Markowska, et al., 1996), and environmental enrichment from 2 to 14 months of age increases cortical and hippocampal NGF concomitant with enhancements in spatial memory (Ickes, et al., 2000, Pham, et al., 1999). It is tempting to speculate that young females who had better performance when young likely had a better cognitive reserve or had greater neuroplasticity, since those that learned better when young had better global novel performance when aged. Hence, increased NGF protein in these animals, instead of solely responding to an age-related insult or a perturbed system (Bimonte, et al., 2003; unpublished observations, Sugaya, et al., 1998), might reflect a beneficial brain environment subserving plasticity and neural network activity (see Levi-Montalcini, 1987).
Using male and female rats, we examined whether age-related cognitive decline can be attenuated by adult lifelong cognitive training.
Cognitive training enhanced performance on the practice task when aged in both sexes, and benefits transferred to novel tasks only in females.
Rats that showed faster learning when young demonstrated better memory when aged.
Neurotrophin levels were related to performance on the training task only in females.
Cognitive training can help cognition later in life, with broader enhancements and associations with neurotrophins in females.
Acknowledgments
This research was supported in part by funds from National Institute on Aging grants awarded to HBN (R03AG026137, R01AG028084), state of Arizona, ADHS and the Arizona Alzheimer's Disease Core Center. We wish to thank Dr. Clark Presson and Dr. Gene Alexander for discussion of this project in thesis form, as well as Dr. Victor Denenberg and Dr. Gordon Winocur for critical feedback and discussion on the experimental design. We thank Donovan Terry and Britny Sundin for excellent experimental assistance.
Footnotes
Disclosure statement: None of the authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joshua S. Talboom, Email: Joshua.Talboom@asu.edu.
Stephen G. West, Email: sgwest@asu.edu.
Elizabeth B. Engler-Chiurazzi, Email: Elizabeth.Engler-Chiurazzi@asu.edu.
Craig K. Enders, Email: Craig.Enders@asu.edu.
Ian Crain, Email: eduicrain@asu.edu.
References
- Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009a;150(9):4248–59. doi: 10.1210/en.2008-1802. en.2008-1802 [pii] 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav. 2009b;55(3):454–64. doi: 10.1016/j.yhbeh.2008.11.008. S0018-506X(08)00315-2 [pii] 10.1016/j.yhbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S, Ohashi Y. Longitudinal study on age-related changes of working and reference memory in the rat. Neuroscience letters. 1991;128(1):17–20. doi: 10.1016/0304-3940(91)90750-n. 0304-3940(91)90750-N [pii] [DOI] [PubMed] [Google Scholar]
- Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, Kong E, Larraburo Y, Rolle C, Johnston E, Gazzaley A. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97–101. doi: 10.1038/nature12486. http://www.nature.com/nature/journal/v501/n7465/abs/nature12486.html#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, A.V.M. AVMA guidelines on euthanasia (formerly Report of the AVMA Panel on Euthanasia) 2007 Jun 2007 update. Available from http://www.avma.org/issues/animal_welfare/euthanasia.pdf.
- Backman C, Rose GM, Hoffer BJ, Henry MA, Bartus RT, Friden P, Granholm AC. Systemic administration of a nerve growth factor conjugate reverses age-related cognitive dysfunction and prevents cholinergic neuron atrophy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16(17):5437–42. doi: 10.1523/JNEUROSCI.16-17-05437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377(9770):1019–31. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Nadel L, Honig WK. Spatial memory deficit in senescent rats. Can J Psychol. 1980;34(1):29–39. doi: 10.1037/h0081022. [DOI] [PubMed] [Google Scholar]
- Bierley RA, Rixen GJ, Troster AI, Beatty WW. Preserved spatial memory in old rats survives 10 months without training. Behav Neural Biol. 1986;45(2):223–9. doi: 10.1016/s0163-1047(86)90794-6. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Granholm AC, Nelson ME, Moore AB. Patterns of neurotrophin protein levels in male and female Fischer 344 rats from adulthood to senescence: how young is “young” and how old is “old”? Exp Aging Res. 2008;34(1):13–26. doi: 10.1080/03610730701761908. 789519642 [pii] 10.1080/03610730701761908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm ACE. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res. 2003a;139(1-2):47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm AC. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci. 2003b;117(6):1395–406. doi: 10.1037/0735-7044.117.6.1395. 10.1037/0735-7044.117.6.1395 2003-10460-025 [pii] [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm AC. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Experimental neurology. 2003c;181(2):301–12. doi: 10.1016/s0014-4886(03)00061-x. S001448860300061X [pii] [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci. 2004;118(4):707–14. doi: 10.1037/0735-7044.118.4.707. 10.1037/0735-7044.118.4.707 2004-16908-005 [pii] [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24(2):161–73. doi: 10.1016/s0306-4530(98)00068-7. S0306-4530(98)00068-7 [pii] [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Sex differences in vicarious trial-and-error behavior during radial arm maze learning. Physiol Behav. 2000;68(4):495–9. doi: 10.1016/s0031-9384(99)00201-2. S0031-9384(99)00201-2 [pii] [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Granholm AC, Seo H, Isacson O. Spatial memory testing decreases hippocampal amyloid precursor protein in young, but not aged, female rats. Neuroscience letters. 2002;328(1):50–4. doi: 10.1016/s0304-3940(02)00442-1. S0304394002004421 [pii] [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70(3-4):311–7. doi: 10.1016/s0031-9384(00)00259-6. S0031-9384(00)00259-6 [pii] [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging. 2003;24(1):37–48. doi: 10.1016/s0197-4580(02)00015-5. S0197458002000155 [pii] [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Friedland RP, Farrer L, Baldwin C, Korczyn A. Genetic and environmental risk factors for Alzheimer's disease in Israeli Arabs. J Mol Neurosci. 2002;19(1-2):239–45. doi: 10.1007/s12031-002-0040-4. JMN:19:1-2:239 [pii] 10.1007/s12031-002-0040-4. [DOI] [PubMed] [Google Scholar]
- Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiology of learning and memory. 2010;93(3):444–53. doi: 10.1016/j.nlm.2010.01.002. S1074-7427(10)00003-1 [pii] 10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang K, Koo EH. Emerging therapeutics for Alzheimer's disease. Annu Rev Pharmacol Toxicol. 2014;54:381–405. doi: 10.1146/annurev-pharmtox-011613-135932. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. L. Erlbaum Associates; Hillsdale, N.J.: 1988. [Google Scholar]
- De Ronchi D, Fratiglioni L, Rucci P, Paternico A, Graziani S, Dalmonte E. The effect of education on dementia occurrence in an Italian population with middle to high socioeconomic status. Neurology. 1998;50(5):1231–8. doi: 10.1212/wnl.50.5.1231. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Sherman GF, Schrott LM, Rosen GD, Galaburda AM. Spatial learning, discrimination learning, paw preference and neocortical ectopias in two autoimmune strains of mice. Brain research. 1991;562(1):98–104. doi: 10.1016/0006-8993(91)91192-4. 0006-8993(91)91192-4 [pii] [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Talgo NW, Schrott LM, Kenner GH. A computer-aided procedure for measuring discrimination learning. Physiol Behav. 1990;47(5):1031–4. doi: 10.1016/0031-9384(90)90031-x. 0031-9384(90)90031-X [pii] [DOI] [PubMed] [Google Scholar]
- Digby GJ, Noetzel MJ, Bubser M, Utley TJ, Walker AG, Byun NE, Lebois EP, Xiang Z, Sheffler DJ, Cho HP, Davis AA, Nemirovsky NE, Mennenga SE, Camp BW, Bimonte-Nelson HA, Bode J, Italiano K, Morrison R, Daniels JS, Niswender CM, Olive MF, Lindsley CW, Jones CK, Conn PJ. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(25):8532–44. doi: 10.1523/JNEUROSCI.0337-12.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30(2):113–21. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- Eckerman DA, Gordon WA, Edwards JD, MacPhail RC, Gage MI. Effects of scopolamine, pentobarbital, and amphetamine on radial arm maze performance in the rat. Pharmacol Biochem Behav. 1980;12(4):595–602. doi: 10.1016/0091-3057(80)90194-x. [DOI] [PubMed] [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, Huentelman MJ, Bimonte-Nelson HA. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.09.005. S0197-4580(09)00311-X [pii] 10.1016/j.neurobiolagin.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Wictorin K, Bjorklund A, Williams LR, Varon S, Gage FH. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature. 1987;329(6134):65–8. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Bimonte HA. Hormones, brain, and behavior: Putative biological contributions to cognitive sex differences. Biology, society, and behavior: the development of sex differences in cognition. 2002;21:55–91. [Google Scholar]
- Fritsch T, McClendon MJ, Smyth KA, Ogrocki PK. Effects of educational attainment and occupational status on cognitive and functional decline in persons with Alzheimer-type dementia. Int Psychogeriatr. 2002;14(4):347–63. doi: 10.1017/s1041610202008554. [DOI] [PubMed] [Google Scholar]
- Gatz M, Svedberg P, Pedersen NL, Mortimer JA, Berg S, Johansson B. Education and the risk of Alzheimer's disease: findings from the study of dementia in Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2001;56(5):P292–300. doi: 10.1093/geronb/56.5.p292. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741–8. doi: 10.1016/S0306-4530(03)00118-5. 10.1016/S0306-4530(03)00118-5 S0306453003001185 [pii] [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Vol. 10. NEURON; CAMBRIDGE MA: 1993. p. 77. [DOI] [PubMed] [Google Scholar]
- Granholm AC. Oestrogen and nerve growth factor - neuroprotection and repair in Alzheimer's disease. Expert Opin Investig Drugs. 2000;9(4):685–94. doi: 10.1517/13543784.9.4.685. [DOI] [PubMed] [Google Scholar]
- Gustilo MC, Markowska AL, Breckler SJ, Fleischman CA, Price DL, Koliatsos VE. Evidence that nerve growth factor influences recent memory through structural changes in septohippocampal cholinergic neurons. The Journal of comparative neurology. 1999;405(4):491–507. doi: 10.1002/(sici)1096-9861(19990322)405:4<491::aid-cne4>3.0.co;2-n. 10.1002/(SICI)1096-9861(19990322)405:4<491::AIDCNE4>3.0.CO;2-N [pii] [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Current opinion in neurobiology. 2006;16(6):710–5. doi: 10.1016/j.conb.2006.09.002. S0959-4388(06)00122-X [pii] 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior; a neuropsychological theory. Wiley; New York: 1949. [Google Scholar]
- Hyde LA, Sherman GF, Hoplight BJ, Denenberg VH. Working memory deficits in BXSB mice with neocortical ectopias. Physiol Behav. 2000;70(1-2):1–5. doi: 10.1016/s0031-9384(00)00239-0. S0031-9384(00)00239-0 [pii] [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Experimental neurology. 2000;164(1):45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Jarrard LE, Okaichi H, Steward O, Goldschmidt RB. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav Neurosci. 1984;98(6):946–54. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- Juraska JM. Sex differences in developmental plasticity of behavior and the brain. Developmental neuropsychobiology. 1986:409–22. [Google Scholar]
- Keppel G, Wickens TD. Design and analysis : a researcher's handbook. 4th. Pearson Prentice Hall; Upper Saddle River, N.J.: 2004. [Google Scholar]
- Koh S, Loy R. Age-related loss of nerve growth factor sensitivity in rat basal forebrain neurons. Brain research. 1988;440(2):396–401. doi: 10.1016/0006-8993(88)91015-3. [DOI] [PubMed] [Google Scholar]
- Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O'Neil JP, Wilson RS, Jagust WJ. Association of Lifetime Cognitive Engagement and Low beta-Amyloid Deposition. Arch Neurol. 2012 doi: 10.1001/archneurol.2011.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkfors L, Ebendal T, Whittemore SR, Persson H, Hoffer B, Olson L. Decreased level of nerve growth factor (NGF) and its messenger RNA in the aged rat brain. Brain research. 1987;427(1):55–60. doi: 10.1016/0169-328x(87)90044-1. [DOI] [PubMed] [Google Scholar]
- Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo JM, Dartigues JF. Are sex and educational level independent predictors of dementia and Alzheimer's disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry. 1999;66(2):177–83. doi: 10.1136/jnnp.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237(4819):1154–62. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Light KR, Kolata S, Wass C, Denman-Brice A, Zagalsky R, Matzel LD. Working memory training promotes general cognitive abilities in genetically heterogeneous mice. Current biology : CB. 2010;20(8):777–82. doi: 10.1016/j.cub.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445–53. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19(18):8122–33. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Koliatsos VE, Breckler SJ, Price DL, Olton DS. Human nerve growth factor improves spatial memory in aged but not in young rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14(8):4815–24. doi: 10.1523/JNEUROSCI.14-08-04815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Price D, Koliatsos VE. Selective effects of nerve growth factor on spatial recent memory as assessed by a delayed nonmatching-to-position task in the water maze. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16(10):3541–8. doi: 10.1523/JNEUROSCI.16-10-03541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Protective effect of practice on cognition during aging: implications for predictive characteristics of performance and efficacy of practice. Neurobiology of learning and memory. 2002;78(2):294–320. doi: 10.1006/nlme.2002.4064. S1074742702940645 [pii] [DOI] [PubMed] [Google Scholar]
- Matzel LD, Light KR, Wass C, Colas-Zelin D, Denman-Brice A, Waddel AC, Kolata S. Longitudinal attentional engagement rescues mice from age-related cognitive declines and cognitive inflexibility. Learn Mem. 2011;18(5):345–56. doi: 10.1101/lm.2034711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Conner JM, Kordower JH. Nerve growth factor in Alzheimer's disease: defective retrograde transport to nucleus basalis. Neuroreport. 1995;6(7):1063–6. doi: 10.1097/00001756-199505090-00028. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.) Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) Guide for the care and use of laboratory animals. National Academies Press; Washington, D.C.: 2011. p. xxv.p. 220. [Google Scholar]
- Ott A, van Rossum CT, van Harskamp F, van de Mheen H, Hofman A, Breteler MM. Education and the incidence of dementia in a large population-based study: the Rotterdam Study. Neurology. 1999;52(3):663–6. doi: 10.1212/wnl.52.3.663. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th. Elsevier Academic Press; Amsterdam ; Boston: 2005. [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–42. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Pham TM, Ickes B, Albeck D, Soderstrom S, Granholm AC, Mohammed AH. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94(1):279–86. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Field MJ, Samson NA, Steventon MJ, Iversen SD. Direct comparison of cognitive facilitation by physostigmine and tetrahydroaminoacridine in two primate models. Neurobiol Aging. 1990;11(6):609–13. doi: 10.1016/0197-4580(90)90025-u. [DOI] [PubMed] [Google Scholar]
- Scali C, Casamenti F, Pazzagli M, Bartolini L, Pepeu G. Nerve growth factor increases extracellular acetylcholine levels in the parietal cortex and hippocampus of aged rats and restores object recognition. Neuroscience letters. 1994;170(1):117–20. doi: 10.1016/0304-3940(94)90253-4. 0304-3940(94)90253-4 [pii] [DOI] [PubMed] [Google Scholar]
- Shimamura A, Berry J, Mangels J, Rustig C. Memory and Cognitive Abilities in University Professors. Psychological Science. 1995;6:271–7. [Google Scholar]
- Shrestha LB. Life expectancy in the United States Congressional Information Service. Library of Congress; 2005. [Google Scholar]
- Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000;33(2-3):199–227. doi: 10.1016/s0165-0173(00)00030-8. S0165017300000308 [pii] [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis : modeling change and event occurrence. Oxford University Press; Oxford ; New York: 2003. [Google Scholar]
- Snowdon DA. Healthy aging and dementia: findings from the Nun Study. Ann Intern Med. 2003;139(5 Pt 2):450–4. doi: 10.7326/0003-4819-139-5_part_2-200309021-00014. 139/5_Part_2/450 [pii] [DOI] [PubMed] [Google Scholar]
- Sugaya K, Greene R, Personett D, Robbins M, Kent C, Bryan D, Skiba E, Gallagher M, McKinney M. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiology of Aging. 1998;19(4):351–61. doi: 10.1016/s0197-4580(98)00072-4. S0197-4580(98)00072-4 [pii] [DOI] [PubMed] [Google Scholar]
- Talboom JS, Engler-Chiurazzi EB, Whiteaker P, Simard AR, Lukas R, Acosta JI, Prokai L, Bimonte-Nelson HA. A component of Premarin((R)) enhances multiple cognitive functions and influences nicotinic receptor expression. Horm Behav. 2010;58(5):917–28. doi: 10.1016/j.yhbeh.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicens P, Carrasco MC, Redolat R. Effects of early training and nicotine treatment on the performance of male NMRI mice in the water maze. Neural Plast. 2003;10(4):303–17. doi: 10.1155/NP.2003.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent GK, Velkoff VA. The next four decades: The older population in the United States: 2010 to 2050. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010. [Google Scholar]
- Warren SG, Juraska JM. Sex differences and estropausal phase effects on water maze performance in aged rats. Neurobiology of learning and memory. 2000;74(3):229–40. doi: 10.1006/nlme.1999.3948. 10.1006/nlme.1999.3948 S1074-7427(99)93948-5 [pii] [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Starr JM, Athawes R, Hunter D, Pattie A, Deary IJ. Childhood mental ability and dementia. Neurology. 2000;55(10):1455–9. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37(6):475–524. doi: 10.1016/0301-0082(91)90006-m. 0301-0082(91)90006-M [pii] [DOI] [PubMed] [Google Scholar]