Abstract
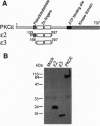
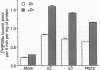
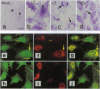
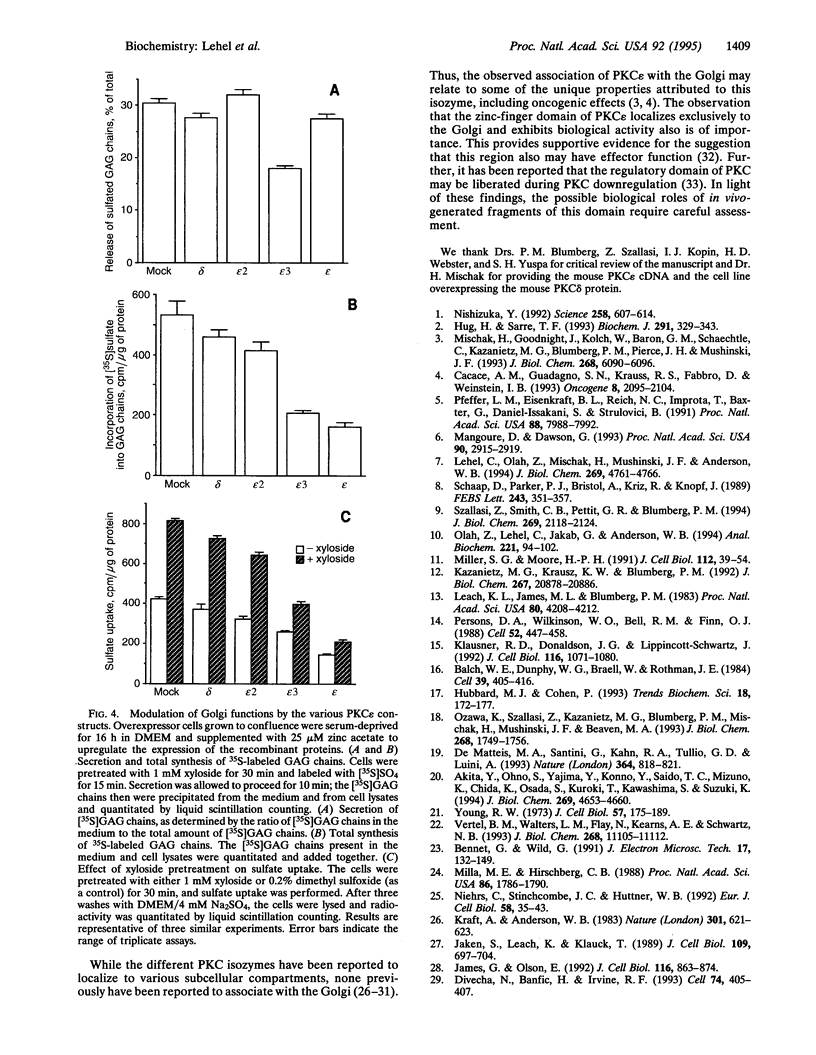
Protein kinase C (PKC) is a multigene family of serine/threonine kinases that are central to many signal transduction pathways. Among the PKC isozymes, only PKC epsilon has been reported to exhibit full oncogenic potential. PKC epsilon also displays unique substrate specificity and intracellular localization. To examine the interrelationship between the biological effects and domain structure of PKC epsilon, NIH 3T3 cells were stably transfected to overexpress different epitope-tagged fragments of PKC epsilon. The overexpressed proteins each contain the epsilon-tag peptide at the C terminus to allow ready detection with an antibody specific for the tag. The holo-PKC epsilon was found to localize with the Golgi network and other compartments, whereas the zinc-finger domain localized exclusively at the Golgi. Golgi-specific glycosaminoglycan sulfation was strongly inhibited in cells overexpressing either holo-PKC epsilon or its zinc-finger domain, while the secretion of sulfated glycosaminoglycans into the medium was impaired in cells expressing the PKC epsilon zinc-finger domain. Thus, these results suggest that PKC epsilon may be involved in specifically regulating Golgi-related processes. Further, the results indicate that PKC epsilon domains other than the kinase domain may also have biological activity and that the zinc-finger domain may function as a subcellular localization signal.
Full text
PDF
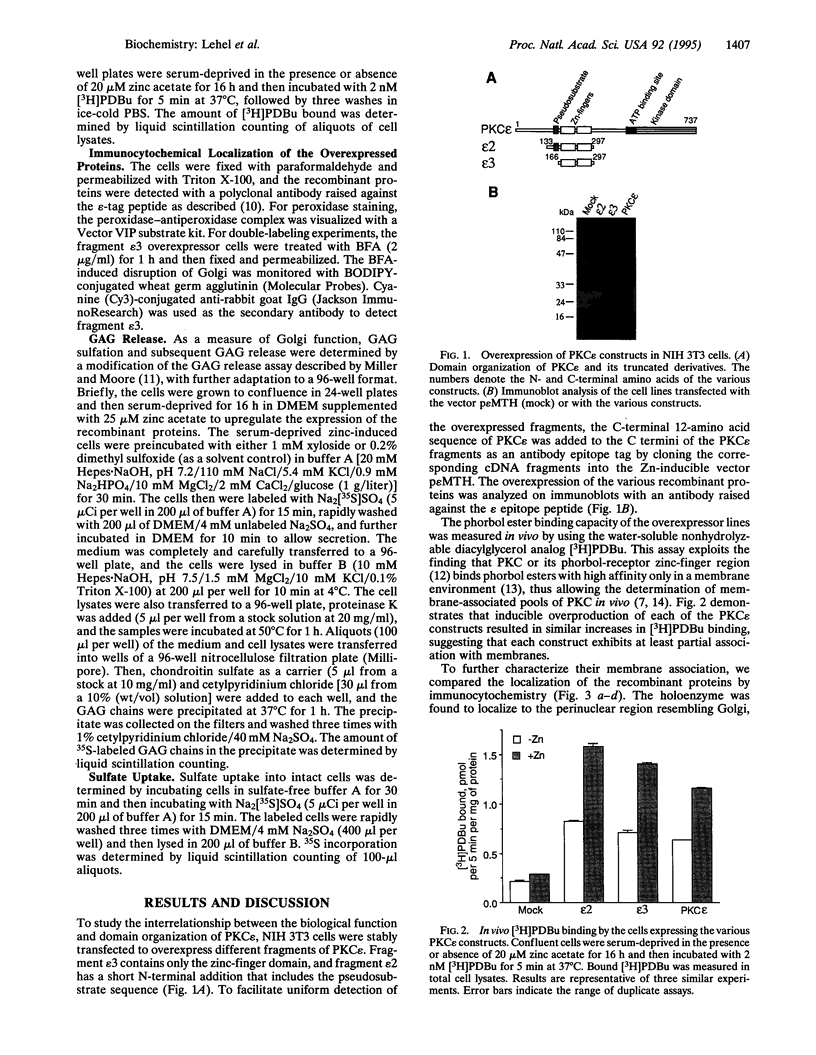


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akita Y., Ohno S., Yajima Y., Konno Y., Saido T. C., Mizuno K., Chida K., Osada S., Kuroki T., Kawashima S. Overproduction of a Ca(2+)-independent protein kinase C isozyme, nPKC epsilon, increases the secretion of prolactin from thyrotropin-releasing hormone-stimulated rat pituitary GH4C1 cells. J Biol Chem. 1994 Feb 11;269(6):4653–4660. [PubMed] [Google Scholar]
- Balch W. E., Dunphy W. G., Braell W. A., Rothman J. E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984 Dec;39(2 Pt 1):405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Bennett G., Wild G. Traffic through the Golgi apparatus as studied by radioautography. J Electron Microsc Tech. 1991 Feb;17(2):132–149. doi: 10.1002/jemt.1060170203. [DOI] [PubMed] [Google Scholar]
- Cacace A. M., Guadagno S. N., Krauss R. S., Fabbro D., Weinstein I. B. The epsilon isoform of protein kinase C is an oncogene when overexpressed in rat fibroblasts. Oncogene. 1993 Aug;8(8):2095–2104. [PubMed] [Google Scholar]
- De Matteis M. A., Santini G., Kahn R. A., Di Tullio G., Luini A. Receptor and protein kinase C-mediated regulation of ARF binding to the Golgi complex. Nature. 1993 Aug 26;364(6440):818–821. doi: 10.1038/364818a0. [DOI] [PubMed] [Google Scholar]
- Divecha N., Banfić H., Irvine R. F. Inositides and the nucleus and inositides in the nucleus. Cell. 1993 Aug 13;74(3):405–407. doi: 10.1016/0092-8674(93)80041-c. [DOI] [PubMed] [Google Scholar]
- Hubbard M. J., Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993 May;18(5):172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- Hug H., Sarre T. F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993 Apr 15;291(Pt 2):329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaken S., Leach K., Klauck T. Association of type 3 protein kinase C with focal contacts in rat embryo fibroblasts. J Cell Biol. 1989 Aug;109(2):697–704. doi: 10.1083/jcb.109.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G., Olson E. Deletion of the regulatory domain of protein kinase C alpha exposes regions in the hinge and catalytic domains that mediate nuclear targeting. J Cell Biol. 1992 Feb;116(4):863–874. doi: 10.1083/jcb.116.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanietz M. G., Krausz K. W., Blumberg P. M. Differential irreversible insertion of protein kinase C into phospholipid vesicles by phorbol esters and related activators. J Biol Chem. 1992 Oct 15;267(29):20878–20886. [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehel C., Olah Z., Mischak H., Mushinski J. F., Anderson W. B. Overexpressed protein kinase C-delta and -epsilon subtypes in NIH 3T3 cells exhibit differential subcellular localization and differential regulation of sodium-dependent phosphate uptake. J Biol Chem. 1994 Feb 18;269(7):4761–4766. [PubMed] [Google Scholar]
- Mangoura D., Dawson G. Opioid peptides activate phospholipase D and protein kinase C-epsilon in chicken embryo neuron cultures. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2915–2919. doi: 10.1073/pnas.90.7.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milla M. E., Hirschberg C. B. Reconstitution of Golgi vesicle CMP-sialic acid and adenosine 3'-phosphate 5'-phosphosulfate transport into proteoliposomes. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1786–1790. doi: 10.1073/pnas.86.6.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. G., Moore H. P. Reconstitution of constitutive secretion using semi-intact cells: regulation by GTP but not calcium. J Cell Biol. 1991 Jan;112(1):39–54. doi: 10.1083/jcb.112.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischak H., Goodnight J. A., Kolch W., Martiny-Baron G., Schaechtle C., Kazanietz M. G., Blumberg P. M., Pierce J. H., Mushinski J. F. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993 Mar 25;268(9):6090–6096. [PubMed] [Google Scholar]
- Niehrs C., Stinchcombe J. C., Huttner W. B. Two membrane-bound forms of tyrosylprotein sulfotransferase as revealed by phase partitioning in Triton X-114. Eur J Cell Biol. 1992 Jun;58(1):35–43. [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Ohno S., Konno Y., Akita Y., Yano A., Suzuki K. A point mutation at the putative ATP-binding site of protein kinase C alpha abolishes the kinase activity and renders it down-regulation-insensitive. A molecular link between autophosphorylation and down-regulation. J Biol Chem. 1990 Apr 15;265(11):6296–6300. [PubMed] [Google Scholar]
- Oláh Z., Lehel C., Jakab G., Anderson W. B. A cloning and epsilon-epitope-tagging insert for the expression of polymerase chain reaction-generated cDNA fragments in Escherichia coli and mammalian cells. Anal Biochem. 1994 Aug 15;221(1):94–102. doi: 10.1006/abio.1994.1384. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Szallasi Z., Kazanietz M. G., Blumberg P. M., Mischak H., Mushinski J. F., Beaven M. A. Ca(2+)-dependent and Ca(2+)-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J Biol Chem. 1993 Jan 25;268(3):1749–1756. [PubMed] [Google Scholar]
- Persons D. A., Wilkison W. O., Bell R. M., Finn O. J. Altered growth regulation and enhanced tumorigenicity of NIH 3T3 fibroblasts transfected with protein kinase C-I cDNA. Cell. 1988 Feb 12;52(3):447–458. doi: 10.1016/s0092-8674(88)80037-0. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Eisenkraft B. L., Reich N. C., Improta T., Baxter G., Daniel-Issakani S., Strulovici B. Transmembrane signaling by interferon alpha involves diacylglycerol production and activation of the epsilon isoform of protein kinase C in Daudi cells. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7988–7992. doi: 10.1073/pnas.88.18.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap D., Parker P. J., Bristol A., Kriz R., Knopf J. Unique substrate specificity and regulatory properties of PKC-epsilon: a rationale for diversity. FEBS Lett. 1989 Jan 30;243(2):351–357. doi: 10.1016/0014-5793(89)80160-7. [DOI] [PubMed] [Google Scholar]
- Spudich A., Meyer T., Stryer L. Association of the beta isoform of protein kinase C with vimentin filaments. Cell Motil Cytoskeleton. 1992;22(4):250–256. doi: 10.1002/cm.970220405. [DOI] [PubMed] [Google Scholar]
- Szallasi Z., Smith C. B., Pettit G. R., Blumberg P. M. Differential regulation of protein kinase C isozymes by bryostatin 1 and phorbol 12-myristate 13-acetate in NIH 3T3 fibroblasts. J Biol Chem. 1994 Jan 21;269(3):2118–2124. [PubMed] [Google Scholar]
- Thomas T. P., Talwar H. S., Anderson W. B. Phorbol ester-mediated association of protein kinase C to the nuclear fraction in NIH 3T3 cells. Cancer Res. 1988 Apr 1;48(7):1910–1919. [PubMed] [Google Scholar]
- Vertel B. M., Walters L. M., Flay N., Kearns A. E., Schwartz N. B. Xylosylation is an endoplasmic reticulum to Golgi event. J Biol Chem. 1993 May 25;268(15):11105–11112. [PubMed] [Google Scholar]
- Young R. W. The role of the Golgi complex in sulfate metabolism. J Cell Biol. 1973 Apr;57(1):175–189. doi: 10.1083/jcb.57.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]