Abstract
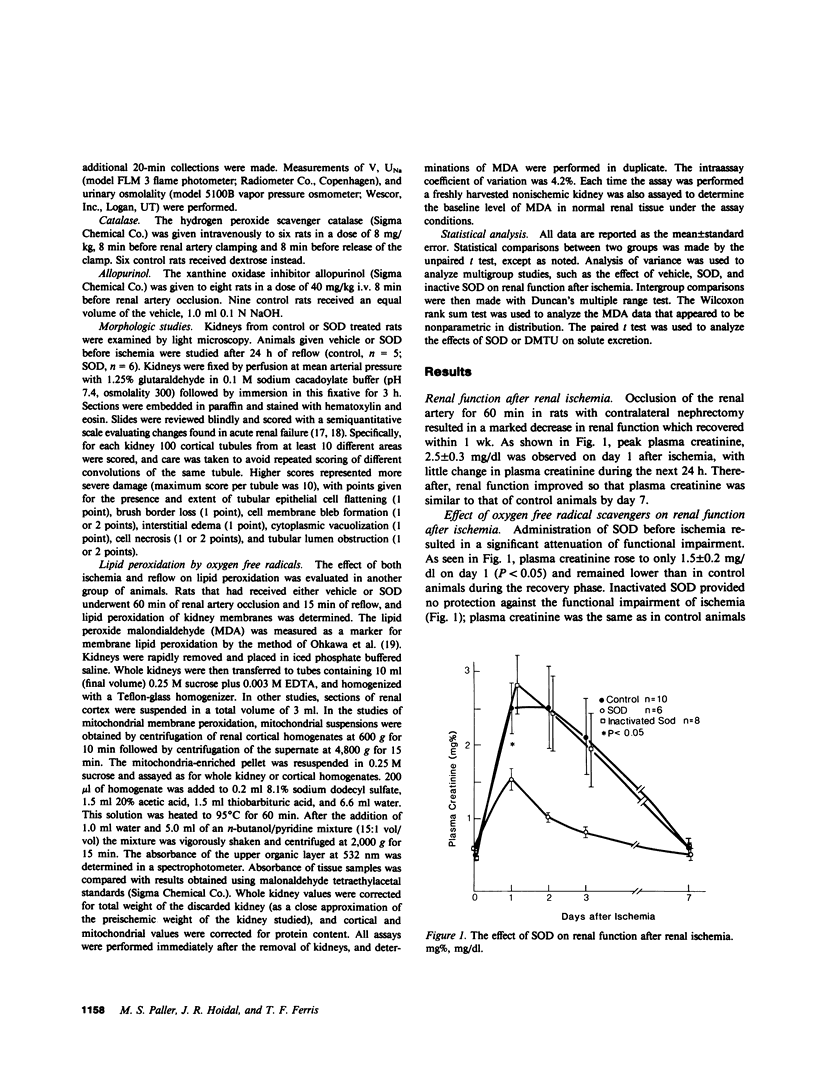
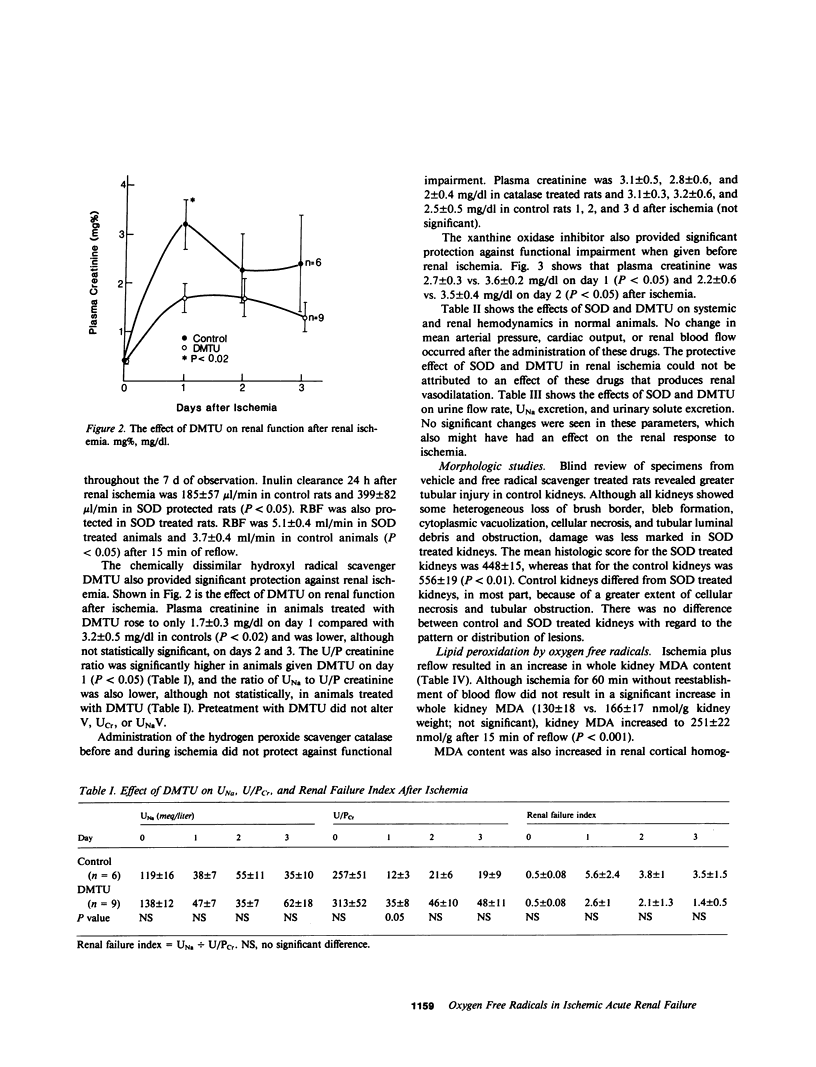
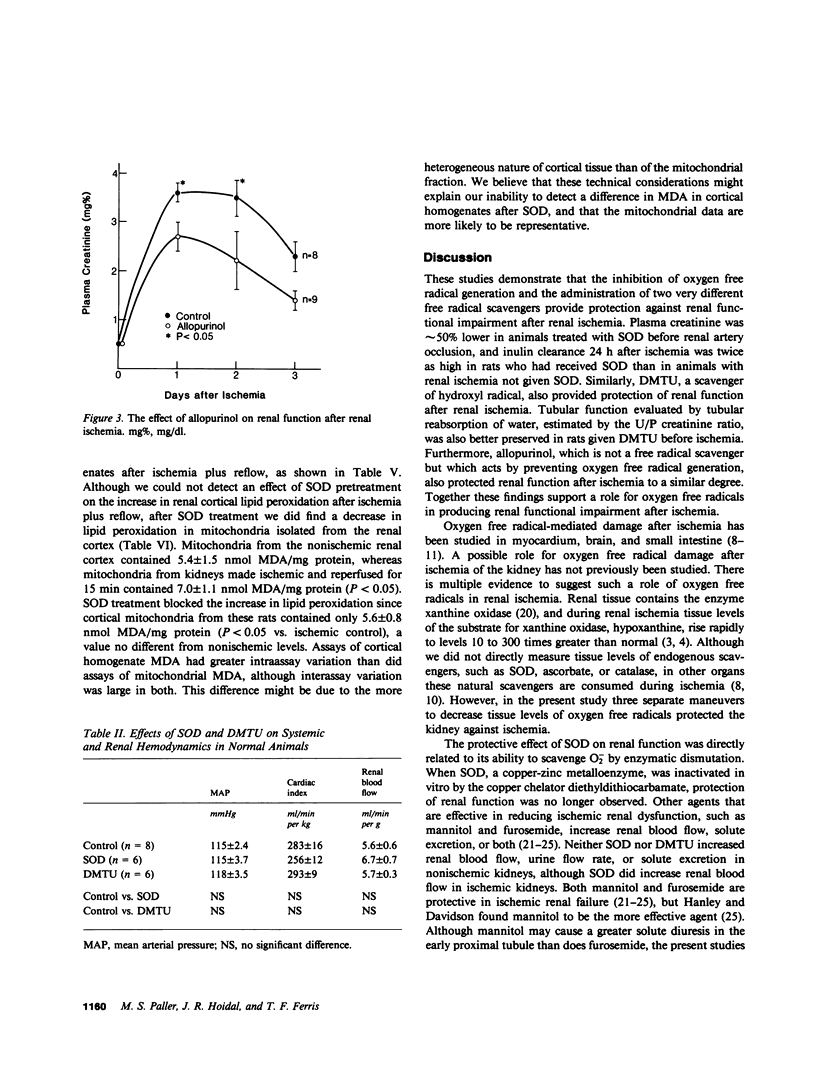
During renal ischemia, ATP is degraded to hypoxanthine. When xanthine oxidase converts hypoxanthine to xanthine in the presence of molecular oxygen, superoxide radical (O-2) is generated. We studied the role of O-2 and its reduction product OH X in mediating renal injury after ischemia. Male Sprague-Dawley rats underwent right nephrectomy followed by 60 min of occlusion of the left renal artery. The O-2 scavenger superoxide dismutase (SOD) was given 8 min before clamping and before release of the renal artery clamp. Control rats received 5% dextrose instead. Plasma creatinine was lower in SOD treated rats: 1.5, 1.0, and 0.8 mg/dl vs. 2.5, 2.5, and 2.1 mg/dl at 24, 48, and 72 h postischemia. 24 h after ischemia inulin clearance was higher in SOD treated rats than in controls (399 vs. 185 microliter/min). Renal blood flow, measured after ischemia plus 15 min of reflow, was also greater in SOD treated than in control rats. Furthermore, tubular injury, judged histologically in perfusion fixed specimens, was less in SOD treated rats. Rats given SOD inactivated by prior incubation with diethyldithiocarbamate had plasma creatinine values no different from those of control rats. The OH X scavenger dimethylthiourea (DMTU) was given before renal artery occlusion. DMTU treated rats had lower plasma creatinine than did controls: 1.7, 1.7, and 1.3 mg/dl vs. 3.2, 2.2, and 2.4 mg/dl at 24, 48, and 72 h postischemia. Neither SOD nor DMTU caused an increase in renal blood flow, urine flow rate, or solute excretion in normal rats. The xanthine oxidase inhibitor allopurinol was given before ischemia to prevent the generation of oxygen free radicals. Plasma creatinine was lower in allopurinol treated rats: 2.7, 2.2, and 1.4 mg/dl vs. 3.6, 3.5, and 2.3 mg/dl at 24, 48, and 72 h postischemia. Catalase treatment did not protect against renal ischemia, perhaps because its large size limits glomerular filtration and access to the tubular lumen. Superoxide-mediated lipid peroxidation was studied after renal ischemia. 60 min of ischemia did not increase the renal content of the lipid peroxide malondialdehyde, whereas ischemia plus 15 min reflow resulted in a large increase in kidney lipid peroxides. Treatment with SOD before renal ischemia prevented the reflow-induced increase in lipid peroxidation in renal cortical mitochondria but not in crude cortical homogenates. In summary, the oxygen free radical scavengers SOD and DMTU, and allopurinol, which inhibits free radical generation, protected renal function after ischemia. Reperfusion after ischemia resulted in lipid peroxidation; SOD decreased lipid peroxidation in cortical mitochondria after renal ischemia and reflow. We concluded that restoration of oxygen supply to ischemic kidney results in the production of oxygen free radicals, which causes renal injury by lipid peroxidation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battelli M. G., Corte E. D., Stirpe F. Xanthine oxidase type D (dehydrogenase) in the intestine and other organs of the rat. Biochem J. 1972 Feb;126(3):747–749. doi: 10.1042/bj1260747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin R. E., de Torrente A., Miller P. D., Bulger R. E., Burke T. J., Schrier R. W. Pathogenic mechanisms in early norepinephrine-induced acute renal failure: functional and histological correlates of protection. Kidney Int. 1978 Aug;14(2):115–125. doi: 10.1038/ki.1978.99. [DOI] [PubMed] [Google Scholar]
- Cunningham C. C., George D. T. The relationship between the bovine heart mitochondrial adenosine triphosphatase, lipophilic compounds, and oligomycin. J Biol Chem. 1975 Mar 25;250(6):2036–2044. [PubMed] [Google Scholar]
- De Torrente A., Miller P. D., Cronin R. E., Paulsin P. E., Erickson A. L., Schrier R. W. Effects of furosemide and acetylcholine in norepinephrine-induced acute renal failure. Am J Physiol. 1978 Aug;235(2):F131–F136. doi: 10.1152/ajprenal.1978.235.2.F131. [DOI] [PubMed] [Google Scholar]
- DeWall R. A., Vasko K. A., Stanley E. L., Kezdi P. Responses of the ischemic myocardium to allopurinol. Am Heart J. 1971 Sep;82(3):362–370. doi: 10.1016/0002-8703(71)90302-4. [DOI] [PubMed] [Google Scholar]
- Demopoulos H. B., Flamm E. S., Pietronigro D. D., Seligman M. L. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol Scand Suppl. 1980;492:91–119. [PubMed] [Google Scholar]
- Deneke S. M., Fanburg B. L. Normobaric oxygen toxicity of the lung. N Engl J Med. 1980 Jul 10;303(2):76–86. doi: 10.1056/NEJM198007103030204. [DOI] [PubMed] [Google Scholar]
- Flamm E. S., Demopoulos H. B., Seligman M. L., Poser R. G., Ransohoff J. Free radicals in cerebral ischemia. Stroke. 1978 Sep-Oct;9(5):445–447. doi: 10.1161/01.str.9.5.445. [DOI] [PubMed] [Google Scholar]
- Flores J., DiBona D. R., Beck C. H., Leaf A. The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J Clin Invest. 1972 Jan;51(1):118–126. doi: 10.1172/JCI106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- GREEN D. E., FLEISCHER S. THE ROLE OF LIPIDS IN MITOCHONDRIAL ELECTRON TRANSFER AND OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1963 Oct 22;70:554–582. doi: 10.1016/0006-3002(63)90793-5. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Guarnieri C., Flamigni F., Caldarera C. M. Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J Mol Cell Cardiol. 1980 Aug;12(8):797–808. doi: 10.1016/0022-2828(80)90081-4. [DOI] [PubMed] [Google Scholar]
- Hanley M. J., Davidson K. Prior mannitol and furosemide infusion in a model of ischemic acute renal failure. Am J Physiol. 1981 Nov;241(5):F556–F564. doi: 10.1152/ajprenal.1981.241.5.F556. [DOI] [PubMed] [Google Scholar]
- Hansson R., Jonsson O., Lundstam S., Pettersson S., Scherstén T., Waldenström J. Effects of free radical scavengers on renal circulation after ischaemia in the rabbit. Clin Sci (Lond) 1983 Dec;65(6):605–610. doi: 10.1042/cs0650605. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970 Nov;120(1):105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- Kogure K., Watson B. D., Busto R., Abe K. Potentiation of lipid peroxides by ischemia in rat brain. Neurochem Res. 1982 Apr;7(4):437–454. doi: 10.1007/BF00965496. [DOI] [PubMed] [Google Scholar]
- Linas S. L., Dickmann D. Mechanism of the decreased renal blood flow in the potassium-depleted conscious rat. Kidney Int. 1982 May;21(5):757–764. doi: 10.1038/ki.1982.94. [DOI] [PubMed] [Google Scholar]
- Maridonneau I., Braquet P., Garay R. P. Na+ and K+ transport damage induced by oxygen free radicals in human red cell membranes. J Biol Chem. 1983 Mar 10;258(5):3107–3113. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mergner W. J., Smith M. A., Trump B. F. Studies on the pathogenesis of ischemic cell injury. IV. Alteration of ionic permeability of mitochondria from ischemis rat kidney. Exp Mol Pathol. 1977 Feb;26(1):1–12. doi: 10.1016/0014-4800(77)90062-4. [DOI] [PubMed] [Google Scholar]
- Mergner W. J., Smith M. W., Trump B. F. Studies on the pathogenesis of ischemic cell injury. XI. P/O ratio and acceptor control. Virchows Arch B Cell Pathol. 1977 Nov 30;26(1):17–26. doi: 10.1007/BF02889532. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Thomas R. A., Berne R. M., Rubio R. Adenosine production in the ischemic kidney. Circ Res. 1978 Sep;43(3):390–397. doi: 10.1161/01.res.43.3.390. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Osswald H., Schmitz H. J., Kemper R. Tissue content of adenosine, inosine and hypoxanthine in the rat kidney after ischemia and postischemic recirculation. Pflugers Arch. 1977 Oct 19;371(1-2):45–49. doi: 10.1007/BF00580771. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Patak R. V., Fadem S. Z., Lifschitz M. D., Stein J. H. Study of factors which modify the development of norepinephrine-induced acute renal failure in the dog. Kidney Int. 1979 Mar;15(3):227–237. doi: 10.1038/ki.1979.30. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Ganote C. E., Jennings R. B. Alterations in renal cortex following ischemic injury. 3. Ultrastructure of proximal tubules after ischemia or autolysis. Lab Invest. 1972 Apr;26(4):347–363. [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel N. J., Glazier W. B., Chaudry I. H., Gaudio K. M., Lytton B., Baue A. E., Kashgarian M. Enhanced recovery from acute renal failure by the postischemic infusin of adenine nucleotides and magnesium chloride in rats. Kidney Int. 1980 Mar;17(3):338–349. doi: 10.1038/ki.1980.39. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Collan Y., Kahng M. W., Trump B. F. Changes in mitochondrial lipids of rat kidney during ischemia. Biochim Biophys Acta. 1980 May 28;618(2):192–201. doi: 10.1016/0005-2760(80)90025-9. [DOI] [PubMed] [Google Scholar]
- Spencer T. L., See J. K., Bygrave F. L. Translocation and binding of adenine nucleotides by rat liver mitochondria partially depleted of phospholipids. Biochim Biophys Acta. 1976 Mar 12;423(3):365–373. doi: 10.1016/0005-2728(76)90193-6. [DOI] [PubMed] [Google Scholar]
- Toledo-Pereyra L. H., Simmons R. L., Najarian J. S. Effect of allopurinol on the preservation of ischemic kidneys perfused with plasma or plasma substitutes. Ann Surg. 1974 Nov;180(5):780–782. doi: 10.1097/00000658-197411000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Pereyra L. H., Simmons R. L., Olson L. C., Najarian J. S. Clinical effect of allopurinol on preserved kidneys: a randomized double-blind study. Ann Surg. 1977 Jan;185(1):128–131. doi: 10.1097/00000658-197701000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A., Bernard D. B., Donohoe J. F., Levinsky N. G. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978 Jul;14(1):31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- Vogt M. T., Farber E. On the molecular pathology of ischemic renal cell death. Reversible and irreversible cellular and mitochondrial metabolic alterations. Am J Pathol. 1968 Jul;53(1):1–26. [PMC free article] [PubMed] [Google Scholar]


