Figure 2. Physiological and pathophysiological regulation of tight junction permeability and passive water transport s by diverse stimuli.
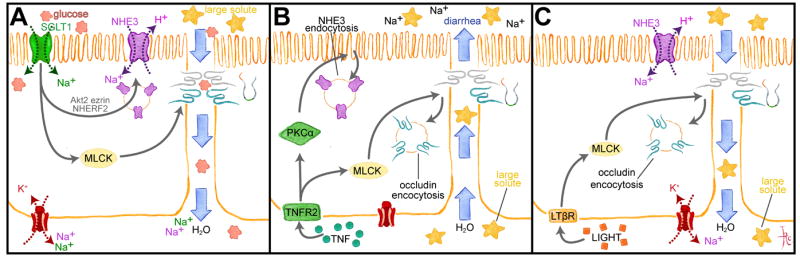
A. SGLT1-dependent Na+ and glucose cotransport triggers a signaling cascade in which Akt2 and MLCK are activated. Akt2 and ezrin promote NHE3 trafficking to the apical membrane to increase transcellular Na+ transport, while MLCK enhances tight junction permeability to small, nutrient-sized molecules, e.g. glucose. Transcellular deposition of Na+ and glucose in the basolateral space creates an osmotic gradient that draws water and glucose across the more permeable paracellular path. B. TNF binds to TNFR2 and activates PKCα and MLCK. NHE3 endocytosis is triggered by PKCα, which reduces transcellular Na+ absorption, thereby reducing the transepithelial Na+ gradient. MLCK activation causes occludin endocytosis that increases tight junction permeability to large solutes, including proteins. These changes result in passive water and solute flow into the lumen. C. Like TNF, LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells) activates MLCK to cause occludin endocytosis and increased tight junction permeability to large solutes. However, LIGHT does not inhibit NHE3, which continues to generate a transepithelial Na+ gradient that enhances passive water absorption.
