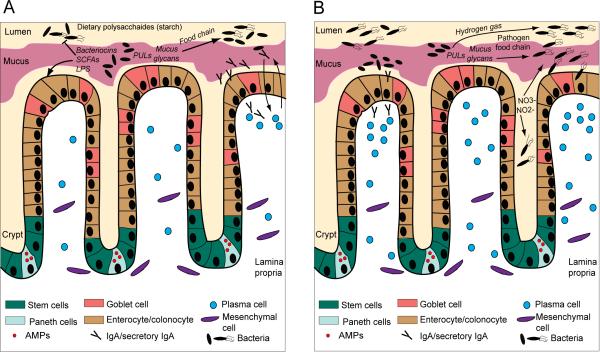
Figure 1. Microbiota exerts control at the epithelial barrier during homeostasis and dysbiosis.
(A) The composition of the epithelial barrier is controlled in the stem cell niche, located in the crypt of Lieberkuhn, giving rise to four cell types. These include Paneth cells (small intestine only) that secrete antimicrobial factors (AMPs), goblet cells secreting glycosylated proteins to generate the protective mucus layer, neuroendocrine cells producing metabolic hormones (not depicted here), and the most abundant cell type the absorptive enterocyte (or colonocyte). A mucus layer covers the epithelial interface with the lumen. During homeostasis commensal microbiota exert effects on the intestinal barrier cells through the production of metabolites by fermentation of dietary polysaccharides such as short chain fatty acids (SCFAs), and the microbial molecular ligands (such as LPS) they express. They also control any potential pathogens and pathobionts through secretion of SCFAs (alter pH, and virulence factor expression) and bacteriocins. Certain commensal microbes possess polysaccharide utilization loci (PULs) that enable the enzymatic harvesting of glycans from mucus. These glycans can then also be sequentially utilized as an energy source by other commensals via a ‘food chain’ mechanism. IgA is produced by plasma cells in the lamina propria and trancytosed across the epithelium as secretory IgA. IgA is induced by and binds to commensals, which creates a reciprocal feedback loop between host-symbiont during homeostasis. IgA also prevents colonization by pathogens and overgrowth of pathobionts. (B) During dysbiosis the commensal microbial community becomes unbalanced and exuberant expansion of pathobionts and colonization and invasion by pathogens may also occur. Invasive flagellated pathogens, such as the Salmonella spp., evade commensal resistance and gain a growth advantage through several mechanisms including utilizing the bacterial products of fermentation (hydrogen gas) and mucus degradation (glycans) in order to produce energy. This could be termed a ‘pathogen food chain’ Commensal E. coli species rapidly outgrow by utilizing nitrate and nitrite ions released by the epithelium during inflammation as terminal electron acceptors to facilitate anaerobic respiration. Outgrowth of microbes and epithelial invasion of pathogens induces increased immune cell recruitment (including IgA-secreting plasma cells) to the epithelial barrier. The reciprocal mechanisms of IgA regulation during dysbiosis are largely unknown, however, defects in the IgA pathway or repertoire can initiate dysbiosis.