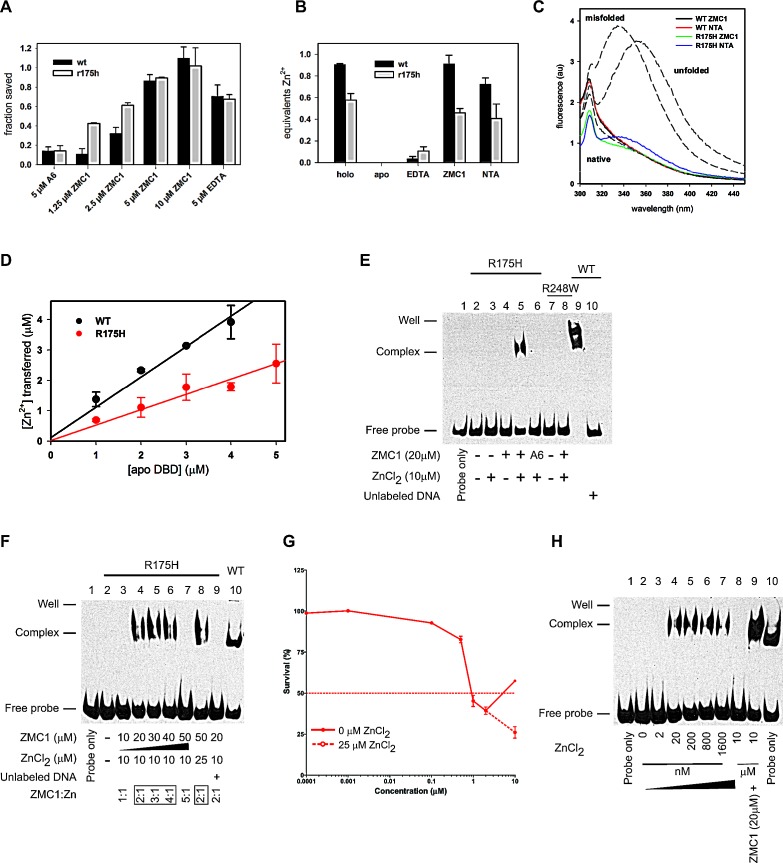
Figure 2. ZMC1 functions as a Zn-metallochaperone.
A, Trp fluorescence used to measure the fraction of R175H and WT DBD saved from Zn2+-arrested refolding. Values are normalized to no treatment and no ZnCl2 controls. Protein was unfolded in 5 M urea, refolded by rapid dilution in the presence of 2.5 μM ZnCl2, then rescued by the indicated treatment. Representative traces available in Supplementary Fig. S2. Error bars are ± SD. B, remetallationof WT and R175H DBD by Zn2+binding compounds. Apo DBD was incubated with ZnCl2 and either EDTA, ZMC1, NTA. The Zn2+ content of the resultant proteins was measured by PAR assay. ZMC1 and NTA can restore Zn2+ to pre-apoization levels. Error bars are ± SD. C, R175H and WT DBD remetallated by ZMC1 and NTA are native by Trp fluorescence. All spectra are of 0.5 μM protein desalted into buffer after the indicated treatment to remove any excess drug or zinc. Gray dashed lines are basis spectra from 0.5 μM WT DBD either freshly purified (native), unfolded in 8 M urea (unfolded), or misfolded with 5 μM ZnCl2 (misfolded). All spectra are similar to native DBD, with the exception of R175H remetallated with NTA, which is suggestive of a small amount of misfolding. D, quantification of Zn2+ transferred from ZMC1 to apo DBDs. The stoichiometries are 1.0 ± 0.1 equivalents (WT) and 0.5 ± 0.1 (R175H). Error bars are ± SD. E, electrophoretic mobility shift assay (EMSA) using WT, R175H and R248W DBD. The WT DBD is used as a positive control (Lane 9). Only the combination of ZMC1 (20μM) and ZnCl2 (10μM) restores site-specific DNA binding to the R175H DBD (Lane 5). The protein-DNA complex is specific because the unlabeled DNA competes for binding (Lane 10). F, EMSA demonstrating the importance of the stoichiometetry of ZMC1:Zn2+for the restoration of DNA binding. G, cell viability assay using serial dilutions of ZMC1. At 10 μM ZMC1, 25 μM ZnCl2 was supplemented. The ZMC1:Zn2+stoichiometric relationship applies to the pharmacodynamics of ZMC1 in p53-R175H cells. H, EMSA demonstrating that remetallation of the apo R175H DBD can occur in the absence of ZMC1. Free zinc of 2 nM to 10 μM and 20 μM ZMC1 were added to the indicated reactions.