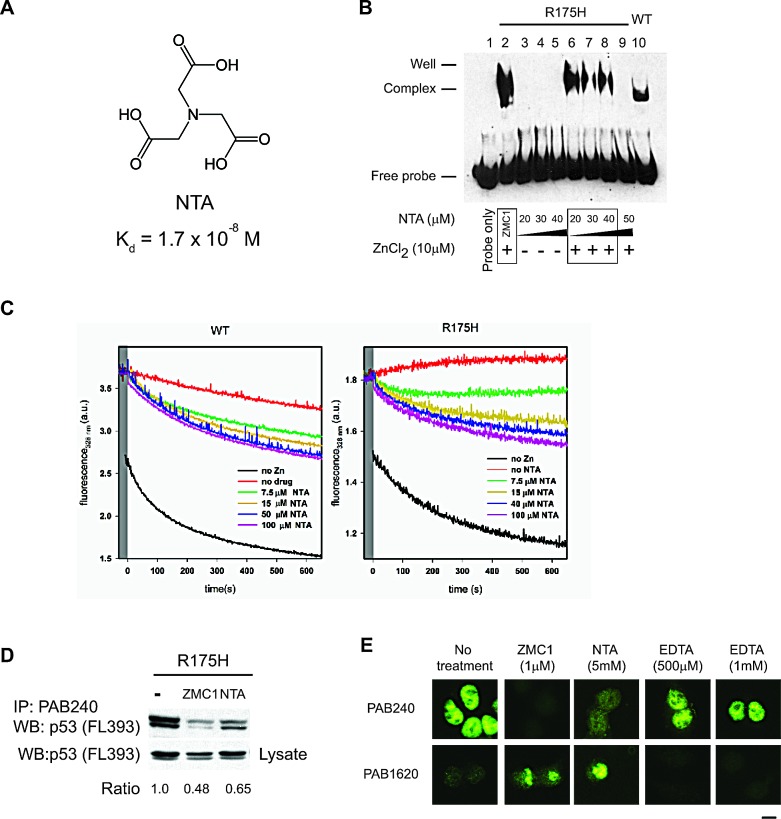
Figure 3. The zinc metallochaperone function is not unique to ZMC1.
A, structure of NTA and its Kd for Zn2+. B, EMSA indicating R175H DBD binds to DNA in the presence of NTA and zinc with the same trend as ZMC1, but not with NTA alone. R175H DBD with ZMC1, zinc and WT DBD are used as positive controls. C, NTA arrested refolding traces. Experiments were run as in Figure S3 but with the indicated concentrations of NTA. NTA was effective at rescuing zinc induced misfolding, but less so than EDTA or ZMC1. D, immunoprecipitation (IP) of p53 protein from R175H cells after treatment of ZMC1 (1 μM) or NTA (5 mM) with the mutant specific PAB240 antibody. The density of western blot bands from IP and lysates were calculated and normalized to no treatment control. E, immunocytochemistry fluorescent staining (IF) of p53 protein from R175H cells after treatment of ZMC1 (1 μM), NTA (5 mM) or EDTA (500 μM or 1 mM). The antibody PAB1620 recognizes WT conformation of p53. Scale bar = 25 μm.