Figure 2. Targeting MUC1-C function suppresses AKT and MEK→ERK signaling.
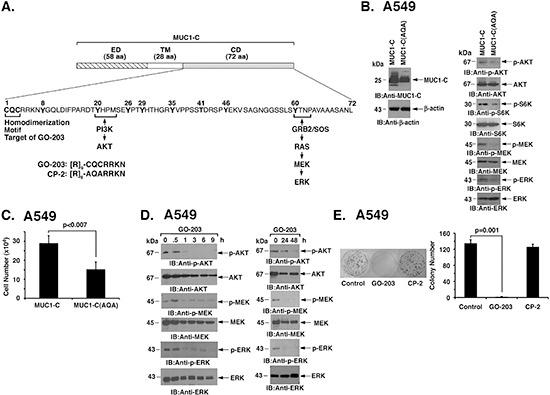
(A) Schema of the MUC1-C subunit with the 58 aa extracellular domain (ED), 28 aa transmembrane domain (TM) and sequence of the 72 aa cytoplasmic domain (CD). The CQC motif is necessary for MUC1-C homodimerization and is the target for GO-203 treatment. Also highlighted are the binding sites that link the MUC1-C cytoplasmic domain to activation of the PI3K→AKT and MEK→ERK pathways. (B) A549 cells were stably transfected with vectors expressing MUC1-C or the MUC1-C(CQC→AQA) mutant [designated MUC1-C(AQA)]. Lysates were immunoblotted with the indicated antibodies. (C) A549/MUC1-C and A549/MUC1-C(AQA) cells were plated at 5 × 104 cells/well. The results (mean±SD of three replicates) are expressed as cell number on day 4. (D) A549 cells were treated with 5 μM GO-203 at 0 and 24 h. Lysates were immunoblotted with the indicated antibodies (left and right). (E) A549 cells were seeded at 1000 cells/well in 6-well plates and left untreated (Control) or treated with 5 μM GO-203 or 5 μM CP-2 each day for 4 days. Colonies were stained with crystal violet on day 15 after treatment (left). Colony number (>30 cells) is expressed as the mean±SD of three replicates (right).
