Figure 7. MUC1-C promotes mutant KRAS NSCLC cell tumorigenicity.
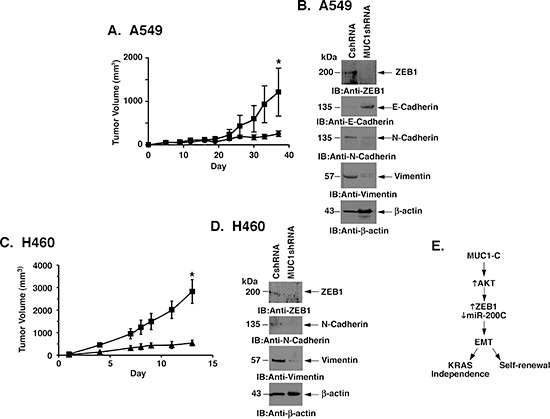
(A) A549/CshRNA (squares) and A549/ MUC1shRNA (circles) cells (4 × 106) were injected subcutaneously in the flanks of female nude mice. Tumor volumes were determined on the indicated days after injection. The results are expressed as tumor volumes (mean±SEM for 3 mice). The asterisk denotes a significant difference (p=0.02) between growth of the A549/CshRNA and A549/MUC1shRNA tumors on day 38. (B) Lysates from tumors isolated on day 30 from mice in the different treatment groups were immunoblotted with indicated antibodies. (C) H460/CshRNA (squares) and H460/MUC1shRNA (triangles) cells (4 × 106) were injected subcutaneously in the flanks of female nude mice. Tumor volumes were determined on the indicated days after injection. The results are expressed as tumor volumes (mean±SEM for 3 mice). The asterisk denotes a significant difference (p=0.013) between growth of the H460/CshRNA and H460/MUC1shRNA tumors on day 13. (D) Lysates from tumors isolated on day 13 from mice in the different treatment groups were immunoblotted with indicated antibodies. (E) Schema depicting the proposed pathway in which MUC1-C activates AKT and thereby the coordinate induction of ZEB1 and suppression of miR-200c. In turn, MUC1-C drives EMT, self-renewal and KRAS independence.
