Figure 3. In vivo treatment with plerixafor sensitizes leukemic blasts to AraC.
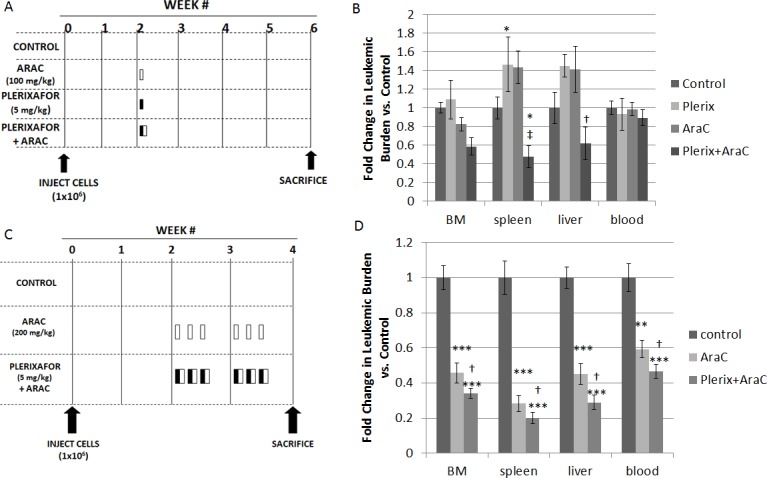
(A) Treatment strategy A. AraC, plerixafor, or vehicle control (phosphate-buffered saline) was administered in a single dose at week 2: AraC was administered via intraperitoneal injection, plerixafor was administered via subcutaneous injection, and PBS was administered via both routes. (B) Leukemic burden in the bone marrow, spleen, liver, and blood: the results of 2 primary sample experiments were pooled. To account for variability in leukemic engraftment between samples, leukemic burden was quantified as the absolute number of cells co-expressing human CD45 and human CD19 and normalized to the average leukemic burden in control mice of each primary sample. (C) Treatment strategy B. AraC, plerixafor and AraC, or vehicle control was administered once daily on three consecutive days for two weeks, starting at week 2: AraC was administered via intraperitoneal injection, plerixafor was administered via subcutaneous injection, and PBS was administered via both routes. (D) Leukemic burden was quantified using the methods described above. The results of 4 primary sample experiments were pooled. BM, bone marrow. *p<0.05, **p<0.01, ***p<0.001 vs. control. †p<0.05, ‡p<0.01 vs AraC.
