Figure 6. Assembly of the MRX complex of the mre11 mutants.
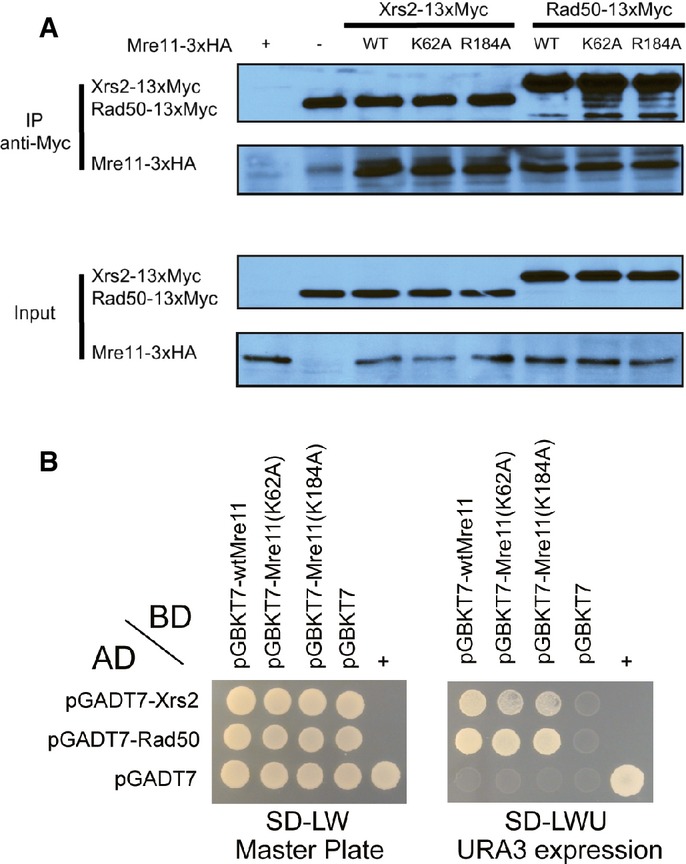
A Co-immunoprecipitation analysis of the interaction between Mre11 or its mutants (K62 and R184A), tagged with 3×HA, and Xrs2 and Rad50, tagged with 13×Myc. Mre11, Rad50, and Xrs2 were pulled down with anti-Myc antibody, and Mre11 proteins were detected using an anti-HA antibody.
B Yeast two-hybrid analysis of Mre11 proteins and other MRX components. Yeast strain (PBN204) was co-transformed with plasmids expressing various BD-Mre11 proteins and other AD-MRX subunits (Rad50 and Xrs2). Transformed yeast cells were spread on selective medium lacking leucine and tryptophan (SD-LW) to select for co-transformants (Master plate). Specific interactions between two proteins were monitored by growth on selective medium lacking leucine, tryptophan, and uracil (SD-LWU). The dimerization of polypyrimidine tract-binding protein served as the positive control (+), and the empty vector pGBKT7 and pGADT7 served as the negative control (−).
Source data are available online for this figure.
