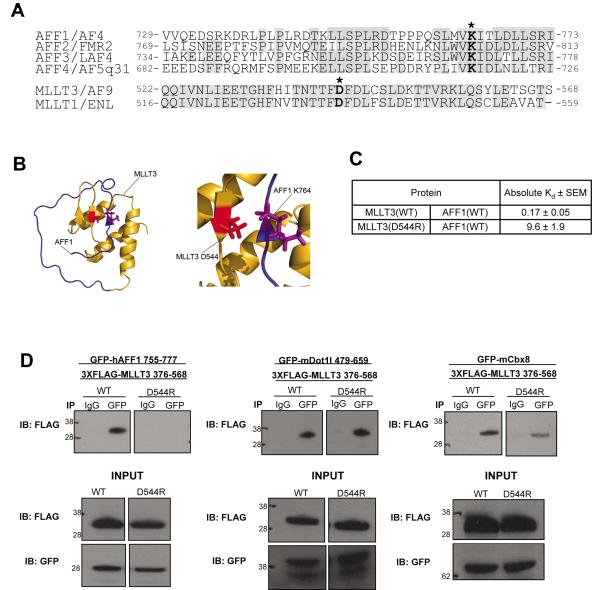
Figure 1. A single electrostatic interaction is a critical determinant for the stable association of AFF1 and MLLT3.
a) Protein sequence alignment of AFF family members shows a high degree of sequence conservation, including AFF1 lysine 764 and AFF4 lysine residue K717 which form a salt bridge with MLLT3 D544 (residues indicated by asterisks). MLLT3 is highly homologous to family member MLLT1. b) Ribbon representation of the structure of the MLLT3 – AFF1 complex (PDB code 2LMO [11]) with the side chains of MLLT3 D544 and AFF1 K764 shown. c) Binding assays were conducted as described in [11]. d) MLLT3(D544R) mutation disrupts the interaction with hAFF1, while maintaining an interaction with both Dot1l and Cbx8.