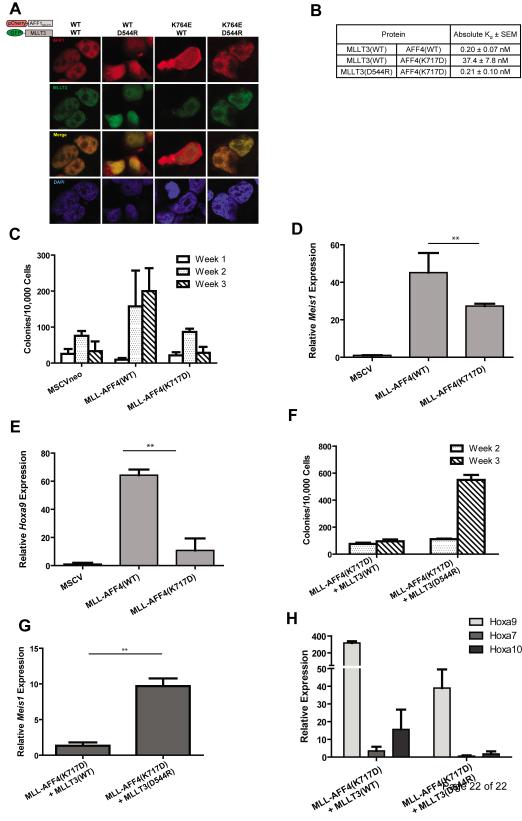
Figure 3. Restoration of the salt-bridge interaction between MLLT3 and AFF1/AFF4 is required for nuclear colocalization and rescue of in vitro leukemic transformation ability.
a) Co-expression of a fragment AFF1 encompassing residues 623-811and MLLT3(WT) in HEK293T cells leads to colocalization of both proteins in the nucleus. Disruption of the salt-bridge interaction by co-expression of AFF1623-811 with MLLT3(D544R), or AFF1623-811(K764E) with MLLT3(WT) results in a loss of this colocalization. Restoration of the salt-bridge interaction by expression of the two mutants AFF1(K764E) and MLLT3(D544R) restores nuclear colocalization. b) AFF4(K717D) reduces binding of AF9 by 187 fold. The AF9(D544R) mutant can restore binding of AFF4(K717D) to wild type levels. c) Cells expressing MLL-AFF4(WT) efficiently re-plated for the duration of the experiment, whereas cells expressing MLL-AFF4(K717D) showed reduced colony formation ability. d) Meis1 expression and e) Hoxa9 was reduced in MLL-AFF4(K717D)-expressing cells compared to MLL-AFF4(WT). ** = p<0.02. f) Cells co-expressing MLL-AFF4(K717D) and MLLT3(D544R) showed increased transformation capacity and g) relative Meis1 expression compared to cells co-expressing MLL-AFF4(K717D) and MLL-MLLT3(WT). h) Cells expressing mutant MLL-AFF4(K717D) in conjunction with wild-type MLLT3, and to a lesser degree mutant MLLT3(D544R), exhibit high-level Hoxa9 expression. Also shown is the expression of other MLL Hox gene targets, Hoxa7 and Hoxa10.