Abstract
Although epidemiological studies provide strong support for demographic and environmental risk factors in psychotic disorders, few data examine how these risk factors relate to the putative aberrant neurodevelopment associated with illness. The present study examined how the accumulation of risk factors including low IQ, low parental socioeconomic status, history of adolescent cannabis use and childhood trauma, and high levels of subclinical psychotic-like experiences contributed to aberrant neurodevelopmental outcomes in 112 otherwise healthy adults recruited from the community. Participants were studied with diffusion tensor imaging, and voxel-wise statistical analysis of fractional anisotropy (FA) using tract-based spatial statistics was used to examine the relation between cumulative risk (CR) for psychosis and white matter (WM) integrity across the whole brain. Analyses revealed that higher CR was significantly associated with lower FA in a cluster in the left superior longitudinal fasciculus. These results suggest that risk factors previously associated with psychotic disorders are associated with WM integrity even in otherwise healthy adults and may provide insight into how previously identified risk factors contribute to the structural brain abnormalities associated with psychotic illness. Prospective longitudinal studies examining the effect of risk factors on the developmental trajectory of brain WM are warranted.
Keywords: Psychosis, Risk factors, Diffusion tensor imaging (DTI), Superior longitudinal fasciculus
1. Introduction
Psychotic disorders such as schizophrenia (SZ) are complex illnesses with typical onset in late adolescence or early adulthood (Cannon et al., 2003). Although several lines of evidence suggest that SZ represents an end state of abnormal neurodevelopment that begins many years before the onset of the illness, the pathophysiology of the disease is not well understood (Rapoport et al., 2012). A range of epidemiological studies, however, provide strong support for myriad antecedent demographic and environmental risk factors in the development of SZ (Matheson et al., 2011). To date few studies have examined how these risk factors relate to the putative aberrant neurodevelopment associated with illness.
Among the most comprehensively studied antecedents to the development of SZ are deficits in intellectual function (Aylward et al., 1984;; Heinrichs and Zakzanis, 1998; Matheson et al., 2011). Data from several birth cohorts (Jones et al., 1994; Kremen et al., 1998; Cannon et al., 2002; Zammit et al., 2004) suggest that low IQ scores are associated with an increased risk for the later development of SZ. Given the complex, and somewhat controversial, association between IQ and socioeconomic status (SES) (Turkheimer et al., 2003), it is not surprising that low SES has also been found to increase risk for SZ (Saha et al., 2005; Saha et al., 2006). Perhaps due to the illness itself, patients with SZ are overrepresented in the lowest social strata (Hollingshead and Redlich 1958; Eaton, 1985; Dohrenwend et al., 1992). However, recent data suggest that low parental SES (PSES) at the time of birth is also associated with an increased risk for the later development of SZ (Werner et al., 2007).
Recently, attention has been focused on the role of cannabis in increasing risk for SZ. Initial data from 40,000 Swedish conscripts followed up for 15 years indicated that individuals who had smoked cannabis by the age of conscription had a two-fold increase in risk of later developing SZ (Andreasson et al., 1987; Zammit et al., 2002). These findings have been replicated in several prospective studies (van Os et al., 2002; Fergusson et al., 2003; Arseneault et al., 2002), and the pooled odds ratios from recent meta-analyses have yielded effect sizes ranging from 1.4 to 2.1 (Henquet et al., 2005; Moore et al., 2007).
Several recent studies have also provided robust evidence for an association between childhood maltreatment and risk for the development of SZ. In a recent meta-analysis (Varese et al., 2012), childhood adversity was substantially related to an increased risk for psychosis with a population attributable risk of ~33%. Moreover, several prospective studies have shown that reverse causation is unlikely to account for this association (Read et al., 2005; Arseneault et al., 2011; Husted et al., 2012; Alemany et al., 2013).
Finally, longitudinal studies have suggested that high levels of psychosis-related symptoms may also predate illness. For example, Chapman and colleagues (1994) initially demonstrated that adolescents who rated high on scales of magical ideation and perceptual aberration had high rates of psychotic outcomes 10 years later. These results have been further replicated in several distinct cohorts (Poulton et al., 2000; Cannon et al., 2002; Hanssen et al., 2005; Welham et al., 2009).
Despite the routine observation that a subset of children who experience multiple risk factors are much more likely to experience adverse psychological outcomes than those with single risk factors (Rutter, 1979; Rutter, 1981), to date there are a paucity of data on the cumulative effects of psychosis-related risk factors. Examination of the effects of multiple risk factors generally relies upon a metric constructed by dichotomizing each risk factor (i.e., 0=no risk, 1=risk) and then summing the total number of risk factors to obtain a measure of cumulative risk (CR). Although alternative metrics, for example, a summary score representing the sum of Z scores for each risk factor, may be more theoretically compelling, several lines of evidence suggest that such methods are problematic due to low statistical power, extreme higher order interaction terms, low robustness, and collinearity among risk factors. In contrast, the CR metric is “more parsimonious and more statistically sensitive and makes no assumptions about the relative strength of the risk factors, or their collinearity” (Evans et al., 2013).
Although there are few data documenting how CR contributes to psychotic illness, at least one study has produced some intriguing results. Zammit and colleagues (2010) recently studied a sample of over 50,000 Swedish conscripts characterized at age 18 for risk factors including low IQ, cannabis use, psychiatric diagnoses, AND disturbed behavior and social relations. This study found that over a 27-year follow-up period, the risk of developing any non-affective psychosis was greater in the presence of two risk factors than in the presence of a single risk factor. Thus, it seems plausible that the accumulation of risk factors contributes to the underlying pathophysiology of psychotic illness.
In recent years, SZ has increasingly been viewed as a disorder of dysconnectivity, in which decreased connection between brain areas is associated with psychotic symptoms. The primary measure derived from diffusion tensor imaging (DTI), fractional anisotropy (FA), is thought to index white matter (WM) integrity and potentially reflects both myelination and organization of fiber tracts that form the basis of inter-regional brain connections. Generally, FA is lower in SZ patients relative to controls (Ellison-Wright and Bullmore, 2009; Patel et al., 2011; Yao et al., 2013). In our recent meta-analysis of WM development in healthy adolescents (Peters et al., 2012), we found significant correlations between age and FA in several predefined WM tracts. A number of studies have demonstrated a disruption in the trajectory of WM development in psychotic and clinical high risk samples (Karlsgodt et al., 2009; Bloemen et al., 2010; Carletti et al., 2012), suggesting that FA might be an ideal target for assessing the effects of risk factors, which are present during childhood and adolescence, on WM development.
The present study sought to examine how the accumulation of risk factors for psychotic disorders might contribute to aberrant neurodevelopmental outcomes in otherwise healthy adults. We used a whole brain DTI method, a powerful tool for examining WM microstructure in vivo (Beaulieu, 2002). Specifically, we used tract-based spatial statistics (TBSS) (Smith et al., 2006) to examine the relation between previously identified risk factors and WM integrity across the whole brain in a large sample of adult controls recruited from the community.
2. Methods
2.1 Participants
The present sample consists of 112 healthy adult volunteers (60 males, 52 females, mean age = 36.07±13.23; age range = 18.47–68.04 years) who were recruited from the general population for a study of subclinical psychosis funded by the National Institute of Mental Health (MH086756). Details regarding the full sample are provided in DeRosse et al. (2014). A total of 38 additional participants were screened for participation in the present study but were not included because they failed to meet all of the inclusion criteria or met some of the exclusion criteria. Exclusion criteria included first-degree family member with a psychotic illness, present or past psychotic or affective disorder diagnosis, active or recent substance abuse (as assessed by urine toxicology testing), contraindications to magnetic resonance imaging, prior psychosurgery, or pregnancy. All participants provided written informed consent to a protocol approved by the Institutional Review Board of the North Shore-Long Island Jewish Health System.
2.2 Diagnostic rule-out
Participants were initially administered the Structured Clinical Interview for DSM-IV, Non-Patient edition (SCID-I/NP) (First et al., 2002) by trained graduate-level research assistants, and absence of past or present affective or psychotic disorder was then determined by consensus with two senior clinicians on the Zucker Hillside Hospital faculty.
2.3. Assessment of risk factors associated with psychotic illness
2.3.1. Estimated IQ
We used the Wide Range Achievement Test-Third Edition-Reading Subtest (WRAT-3) as an estimate of IQ (Kremen et al., 2006). Although the WRAT-3 may underestimate full-scale IQ, it tends to be very accurate when used to classify individuals with below average intelligence (Griffin et al., 2002).
2.3.1. Parental socioeconomic status (PSES)
PSES was determined using the Hollingshead and Redlich Two-Factor Social Position Index (Hollingshead, 1975), which is based on measures of educational attainment and occupational prestige. PSES is rated on the social position index (SPI) and classifies individuals into one of five potential classes ranging from the highest (Class I) to lowest (Class V) socioeconomic classes. In the present study, we used the rating for the parent that produced the highest class as the primary measure of PSES.
2.3.2. History of cannabis use
History of cannabis use was defined as using cannabis more than once before the age of 18 as assessed with the substance abuse module of the SCID-I/NP (First et al., 2002). None of the participants in the present study met diagnostic criteria for a cannabis use disorder (although that was not an exclusion criterion for the study).
2.3.3. Childhood trauma
To assess the history of childhood trauma, we used the 28-item Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003). The CTQ is a 5-point Likert-type self-report questionnaire that measures several dimensions of trauma during childhood, including physical, emotional and sexual abuse as well as emotional and physical neglect. Summing across all of these dimensions provides a total score that represents the severity of overall trauma experienced by an individual during childhood. The present study used the total score derived from the CTQ.
2.3.4. Subclinical psychotic-like experiences (PLEs)
PLEs were assessed using the Community Assessment of Psychic Experiences (CAPE) (Stefanis et al., 2002). The CAPE is a 42-item self-report questionnaire that measures three dimensions of subclinical psychosis including positive, negative and depressive symptoms. Although the CAPE provides scores on each of these three dimensions, we focused a priori on the overall presence of PLEs. Thus, we used the score resulting from summing the scores on the negative and positive subscales to provide an overall measure of severity of positive and negative subclinical symptoms. It should be noted that in the present sample the positive and negative symptom domains are significantly correlated (r=0.66, p<0.001).
2.4. Cumulative risk (CR) calculation
We used a composite metric of CR (Evans et al., 2013) calculated as the sum of dichotomized risk factors (present vs. absent). To characterize participants by the presence or absence of each risk factor described above, with the exception of history of cannabis use, we divided the sample into quartiles and selected the most extreme quartile to represent the risk group in that domain. If participants fell into the upper quartile for a risk factor, they were considered as having that risk factor and coded as 1 on that factor. Those falling in the lower three quartiles were coded as 0. In the case of cannabis use, any participant who endorsed using cannabis before age 18 was coded as 1, and those who denied using cannabis were coded as 0. We then calculated the CR for each participant by summing the total number of putative risk factors that were present. Thus, CR could range from 0, corresponding to individuals who did not fall into the highest quartile of any of the assessed risk factors and who never used cannabis, to 5, corresponding to individuals who fell into the highest quartile on every risk factor and had used cannabis before the age of 18.
2.5. DTI acquisition and preprocessing
All subjects underwent DTI at the North Shore University Medical Center, Manhasset, NY, on a GE Signa HDx 3.0 T system (General Electric, Milwaukee, WI). The sequence included volumes with diffusion gradients applied along 31 non-parallel directions (b = 1000 s/mm2) and 5 volumes without diffusion weighting (repetition time = 14 s, echo time = min, matrix = 128 × 128, field of view = 240 mm). Each volume consisted of 51 contiguous 2.5-mm axial slices acquired parallel to the anterior-posterior commissural line using a ramp sampled, double spin-echo single shot echo-planar imaging method. We applied rigorous control for motion effects to all scans in accordance with our published studies (Peters et al., 2013; Peters et al., 2014; Argyelan et al., 2013).
A radiologist reviewed all scans, and all images were visually inspected to ensure that no gross abnormalities were evident. Image processing was conducted using the FMRIB Software Library (FSL; Oxford, United Kingdom). Eddy-current distortions and head-motion displacements were corrected through affine registration of the 31 diffusion volumes to the first b0 volume using FSL’s Linear Registration Tool (FLIRT) (Jenkinson and Smith, 2001). The b-vector table (i.e., gradient directions) for each participant was then adjusted according to the rotation parameters of this linear correction. Non-brain tissue was removed using FSL’s Brain Extraction Tool. Fractional anisotropy (FA) and diffusivity parameters, putative measures of neural fiber coherence, diameter and myelination (Beaulieu, 2002), were then calculated at each voxel of the brain by fitting a diffusion tensor model to the raw diffusion data using weighted least squares in FSL’s Diffusion Toolbox.
2.6. Tract-based spatial statistics
Voxel-wise statistical analysis of the FA data was carried out using TBSS in FSL (Smith et al., 2006). All subjects' FA data were aligned to the FMRIB58 FA standard brain using the nonlinear registration tool FNIRT (Smith et al., 2006). Next, an average FA image was created and the tracts were narrowed to generate an FA “skeleton” representing the center of all tracts common to the entire group. The area around the skeleton in each subject’s individual aligned FA map was searched and the highest local FA value was assigned to the skeleton. This ensured that each subject’s skeleton was in the group space, yet represented the center of that subject’s own unique fiber tracts. The FA threshold for the mean FA skeleton was set at 0.2. The registration parameters obtained for the FA maps (i.e., nonlinear warps and subsequent skeleton “projections”) were then also applied to the diffusivity maps.
2.7. Statistical analysis
To test for local association between cumulative risk (CR) and FA, linear regressions on FA (mean-centered) were performed using permutation-based testing with age and sex as covariates (mean-centered). Inference on the statistic maps was carried out using threshold-free cluster enhancement (TCFE) (Smith et al., 2009). The null distribution was built up over 5000 random permutations across the image. The clusters were then thresholded at a level of p<0.05, corrected for multiple comparisons (family-wise error). Anatomical location of significant FA clusters was determined with the probabilistic cortical, subcortical and WM tractography atlases provided in FSL, and an MRI atlas of human WM anatomy (Oishi et al., 2010).
For any FA cluster significantly related to CR, we performed a series of additional analyses in SPSS (version 11.5) to more closely examine the nature of the relationship identified. First, we extracted the mean FA, axial diffusivity (AD, (L2+L3)/2 often considered to index tract organization) and radial diffusivity (RD, L1 often considered to index myelination) within the significant clusters. Next, we carried out correlational analyses to examine the relationships between mean FA, AD and RD and CR as well as the raw scores on our measures of estimated IQ, parental SES, childhood trauma and PLEs. To further examine the contributions of individual risk factors, we performed as series of analyses of covariance (ANCOVAs) controlling for sex and age, comparing mean FA, AD and RD values in any significant cluster for participants with and without each risk factor. Finally, a stepwise multiple regression analysis, controlling for sex and age, was carried out to examine how the raw scores of each risk factor fit into a model predicting overall FA in any significant cluster.
3. Results
Initial comparison of participants across the range of cumulative risk (CR) revealed no significant differences in sex, age or race (p>0.05). The demographics of the overall sample stratified by CR are shown in Table 1. Comparisons of participants characterized on each risk factor are shown in Table 2. Comparison of groups dichotomized for each risk factor individually indicated that those in the most extreme high quartiles on history of childhood trauma and PLEs were significantly older than those in the lower quartiles (t=3.37, p=0.001 and t=2.38, p=0.02, respectively). No differences in age were observed for groups dichotomized on estimated IQ, PSES or cannabis use. The proportion of African American and other minorities was higher than the proportion of Caucasians in the most extreme low quartile of PSES (χ2=9.00, p=0.01) and PLE’s (χ2=6.68, p=0.03). No other differences in age, sex or race were identified.
Table 1.
Demographic characteristics of healthy individuals characterized for the number of putative risk factors, or cumulative risk (CR), for psychotic disorders.
| Cumulative risk | N | Mean age (SD) | Sex | White |
Race Black |
Other |
|---|---|---|---|---|---|---|
| 0 | 24 | 30.70 (9.70) | 13M/11F | 18 | 2 | 4 |
| 1 | 34 | 33.86 (12.41) | 18M/16F | 23 | 6 | 5 |
| 2 | 32 | 38.17 (14.63) | 17M/15F | 14 | 13 | 5 |
| 3 | 17 | 42.06 (12.86) | 9M/8F | 10 | 4 | 3 |
| 4 | 4 | 43.22 (19.05) | 2M/2F | 3 | 1 | 0 |
| 5 | 1 | 42.41 (0) | 1M/0F | 0 | 1 | 0 |
Table 2.
Mean scores and standard deviations (SD) for each putative risk factor in risk present vs. risk absent groups.
|
Risk factor Absent |
Present | Statistic | p value | |
|---|---|---|---|---|
| Mean standardized WRAT-3 score (SD) | 105.75 (5.81) | 88.80 (6.55) | t=12.49 | <0.001 |
| Mean social position Index (SD) | 1.7 (0.46) | 3.47 (0.74) | t=14.72 | <0.001 |
| Mean CTQ total score (SD) | 31.19 (2.42) | 42.73 (8.51) | t=11.07 | <0.001 |
| Mean psychotic-like experiences (SD) | 37.50 (2.83) | 48.21 (4.50) | t=14.79 | <0.001 |
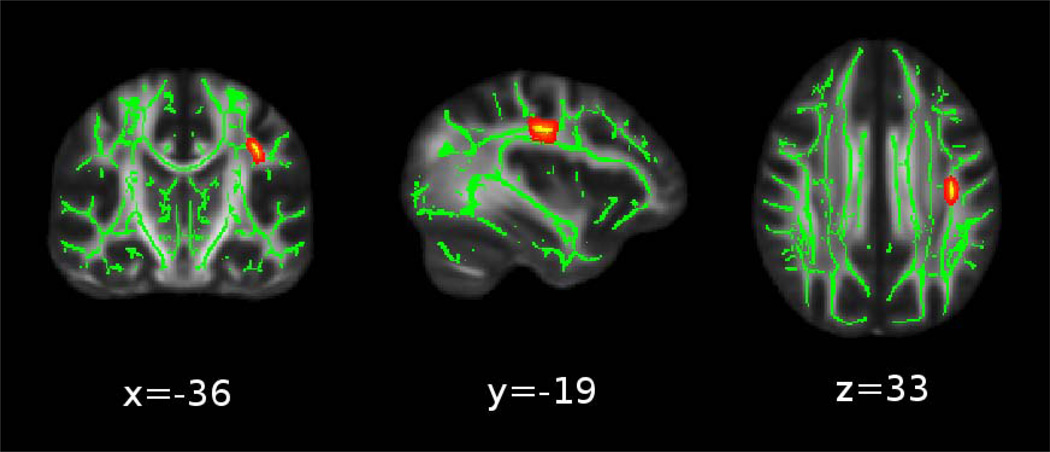
The primary analysis, voxel-wise statistical analysis of FA using TBSS that included age and sex as covariates, revealed that higher CR was significantly predictive of lower FA in a cluster in the left superior longitudinal fasciculus (SLF) (p=0.04, corrected; 118 voxels, peak voxel at x=−36, y=−19, z=33 in FMRIB58 standard space). These data are shown in Fig. 1. CR was not significantly associated with higher FA values in any cluster.
Figure 1.
Cumulative Risk (CR) is associated with lower fractional anisotropy (FA) in the left superior longitudinal fasciculus. The significant clusters (red-yellow) in the FA images are “thickened” into the local tracts by the tbss-fill tool for display purposes, and overlaid on the TBSS skeleton in green. (Note: image is left-to-right).
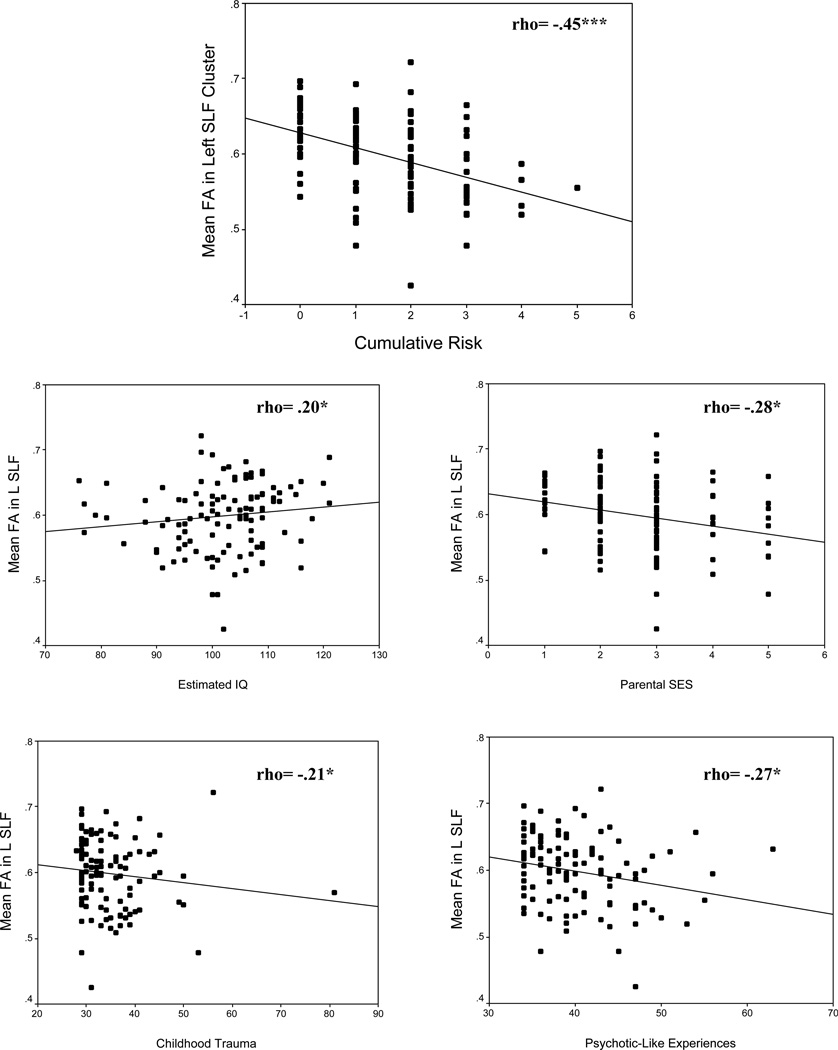
Analyses carried out to examine the relationships between mean FA in the cluster in the left SLF and CR, as well as the raw scores on our measures of estimated IQ, PSES, childhood trauma and PLEs, yielded a consistent pattern of results. Specifically, CR and the raw scores for PSES, history of childhood trauma and PLEs showed a significant negative correlation with FA, and estimated IQ showed a significant positive correlation with FA. These relations are shown in Fig. 2. We also carried out analyses to examine the relationships between mean AD and RD in the cluster in the left SLF and CR, as well as the raw scores on our measures of estimated IQ, PSES, childhood trauma and PLEs. These analyses also yielded a consistent pattern of results. Specifically, CR as well as the raw scores for PSES and PLEs revealed significant negative correlations to AD and significant positive correlations to RD. History of childhood trauma revealed similar associations, although the negative correlation between childhood trauma and AD was not statistically significant. Moreover, estimated IQ showed a significant positive correlation with AD yet also showed a nominal negative correlation with RD. It should be noted, however, that only the correlations between CR and our measures of WM integrity remained significant after corrections for multiple comparisons. These data are shown in supplementary Figs. S1 and S2.
Figure 2.
Relations between mean fractional anisotropy (FA) in the left superior longitudinal fasciculus (SLF) cluster and cumulative risk (CR) (top panel) and individual risk factors (bottom panels). Uncorrected p values are indicated as: *p<.05; **p<.01; ***p<.001.
Note: Effect sizes may be inflated due to the voxel-wise selection of the most significant peak (see Krirgeskorte et al 2009).
Analyses carried out to examine the contributions of individual risk factors to FA in the cluster in the left SLF (a series of ANCOVAs comparing groups dichotomized for each factor and controlling for sex and age) revealed significant associations between FA and low PSES (F(1,108)=7.52, p<0.01) and high levels of PLEs (F(1,108)=11.23, p<0.01). Although there also appeared to be relations between FA in this region and both estimated IQ and history of childhood trauma, these results were not statistically significant (p=0.13 and p=0.15, respectively).
A stepwise multiple regression analysis, correcting for age and sex, carried out to examine how each risk factor fit into a model predicting mean FA in the significant SLF cluster revealed that the full model was significant (F(7,105)=4.83, p<0.001) and accounted for ~20% of the variance in FA within the cluster (r2=0.19). Similar to the ANCOVA described above, the significant predictors in the final model included low PSES (β= −0.03, p<0.01) and high levels of PLEs (β= −0.04, p<0.001).
Finally, we sought to examine whether these relations were still evident when we excluded participants who might still be in the age range for the onset of a psychotic disorder. Thus, we re-ran the stepwise regression in only those participants >30 years old (n=59). This analysis yielded results nearly identical to those obtained in the full sample (r2=0.14)
4. Discussion
The present results suggest that the accumulation of risk factors for psychosis, including low IQ, low PSES, high levels of childhood trauma and PLEs, and history of cannabis use, may have a significant impact on WM integrity within the SLF in otherwise healthy adults. Specifically, we detected a significant negative correlation between the number of putative risk factors carried by an individual and FA in the left SLF. Further, although each risk factor was independently correlated to FA in the left SLF cluster, the relation between cumulative risk (CR) and FA does not appear to be driven by a single risk factor. On the contrary, the association between CR and FA was much stronger than those between FA and any of the risk factors individually, suggesting that these risk factors may interact to affect WM integrity in the SLF. Indeed, in all of the correlational analyses, only the relation between CR and indices of WM integrity remained significant after correction for multiple comparisons.
These findings are particularly noteworthy as several DTI studies, which have found FA increases in the SLF from childhood to early adulthood, have suggested that this tract may play a critical role in the onset of SZ (Lebel et al., 2008; Giorgio et al., 2010; Verhoeven et al., 2010; Peters et al., 2012). Moreover, several earlier studies have found reduced SLF FA in clinical high-risk samples (Karlsgodt et al., 2009; Bloemen et al., 2010; Carletti et al., 2012) and adult patients (Karlsgodt et al., 2008; Peters et al., 2010; Luck et al., 2011). Critically, however, the average age of participants in the present sample was beyond the average age of risk for a psychotic disorder. Thus, the observed association between CR and reduced FA in the SLF is unlikely to be related to risk for psychotic illness. Rather, the present findings may indicate that demographic and environmental factors previously implicated in risk for SZ may play a significant role in the microstructural integrity of this tract independently of risk for SZ. Indeed, reduced FA in the SLF has been reported in adolescent substance abusers (Jacobus et al., 2009; Bava et al., 2009) as well as in adults with substantial histories of childhood trauma (Fani et al., 2012). The convergence of these data might suggest that previously identified risk factors act by negatively impacting brain development in regions that have already been compromised in individuals who are at high risk for developing a psychotic illness; in other words, these previously identified risk factors may be acting to “add insult to injury”.
The pattern of results in the present study related to FA, AD and RD may indicate that changes in axonal fiber coherence and/or diameter as well as myelin integrity play a role in this process (Song et al., 2005; Hofling et al., 2009). RD indexes water diffusion perpendicular to the axon bundles, and increased RD has been found in mouse models of WM demyelination (Song et al., 2005; Hofling et al., 2009). Thus, RD might serve as a putative marker of the effects of CR on myelination and/or myelin integrity. In contrast, AD indexes diffusion parallel to the axons, and decreased AD has been found in axonal damage (Hofling et al., 2009) and, as such, may be a marker of the effects of CR on the coherence of axon bundles, axon diameter and/or axonal integrity (Beaulieu, 2002).
Several limitations should be noted. First, our measure of cumulative risk (CR) was derived from the estimated IQ, PSES, childhood trauma and PLE measures within the overall sample. It is possible that this sample is not representative of the general population. However, previous reports linking individual demographic and environmental factors to risk for SZ are not amenable to the identification of cut-off scores. For example, reports on the relation between premorbid IQ and SZ generally rely on Z scores that are calculated relative to a comparable healthy sample rather than on a standardized IQ value (Reichenberg, 2005). Additionally, our measure of cannabis use before the age of 18 did not allow us to specifically quantify usage pattern. Moreover, although the CR metric may be a more parsimonious method for examining the effects of multiple risk factors, it assumes that risk is linearly additive, and it therefore may result in arbitrary assignment of the presence or absence of risk factors. However, in the absence of well-validated explanatory models for the interaction among risk factors, the CR metric, which makes no assumptions about these interactions, was believed to be best suited for the current study.
It should also be noted that the sample in this study encompassed a broad age range and, as seen in Table 1, the mean age of participants increases as the CR score increases. Thus, it could be argued that the observed effects of CR on FA in the left SLF may be due to age. However, age was included as a covariate in all of our analyses, and in the primary analysis of the effect of CR on FA, age was not a significant predictor of FA in the left SLF (p=0.36). Moreover, the variance accounted for by the risk factors when we limited our analyses to only those participants over the age of 30 was similar to what was observed in the full sample. The present sample also contained a significant proportion of racial minorities and African Americans were more likely to be classified as having the risk factors of low PSES and high levels of PLEs. However, to assess the influence of race on the association between CR and FA in the left SLF, we re-ran the linear regression including race as a covariate in addition to age and sex. In this analysis, race was not a significant covariate (p=0.31) and the relation between CR and FA did not change.
In summary, the present findings may provide some insight into the mechanism by which previously identified risk factors contribute to structural brain abnormalities presumed to underlie psychotic illness. Prospective longitudinal studies examining the developmental trajectory of brain WM in relation to individual risk factors such as the onset of cannabis use, childhood trauma, and the combined effects of these risk factors are warranted. Further, the examination of these effects in the context of recently identified psychosis-related risk variants (Ripke et al., 2011) may provide novel insights into the pathophysiology of psychotic illnesses.
Supplementary Material
Demographic and environmental factors are known to increase risk for schizophrenia
Few studies have examined how these risk factors may affect white matter development
Using TBSS we tested the association between FA and cumulative risk for schizophrenia
We found that cumulative risk was significantly associated with lower FA in the left SLF
These findings may help explain the relation between risk factors and schizophrenia
Acknowledgments
Funding/support
This work was supported by grants from the National Institute of Mental Health to Dr. DeRosse (MH086756), Dr. Szeszko (R01 MH076995), Dr. Malhotra (MH079800), the NSLIJ Research Institute General Clinical Research Center (M01 RR018535), Advanced Center for Intervention and Services Research (P30 MH090590) and a Center for Intervention Development and Applied Research (P50 MH080173).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alemany S, Goldberg X, Van Winkel R, Gastó C, Peralta V, Fananas L. Childhood adversity and psychosis: examining whether the association is due to genetic confounding using a monozygotic twin differences approach. European Psychiatry. 2013;28:207–212. doi: 10.1016/j.eurpsy.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Andréasson S, Engström A, Allebeck P, Rydberg U. Cannabis and schizophrenia: A longitudinal study of Swedish conscripts. The Lancet. 1987;330:1483–1486. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Fisher HL, Polanczyk G, Moffitt TE, Caspi A. Childhood trauma and children's emerging psychotic symptoms: a genetically sensitive longitudinal cohort study. American Journal of Psychiatry. 2011;168:65–72. doi: 10.1176/appi.ajp.2010.10040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyelan M, Ikuta T, DeRosse P, Braga RJ, Burdick KE, John M, Kingsley PB, Malhotra AK, Szeszko PR. Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophrenia Bulletin. 2014;40:100–110. doi: 10.1093/schbul/sbt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward E, Walker E, Bettes B. Intelligence in schizophrenia: meta-analysis of the research. Schizophrenia Bulletin. 1984;10:430–459. doi: 10.1093/schbul/10.3.430. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Research: Neuroimaging. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR in Biomedicine. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bloemen OJN, De Koning MB, Schmitz N, Nieman DH, Becker HE, de Haan L, Dingemans P, Linszen DH, van Amelsvoort TAMJ. White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychological Medicine. 2010;40:1297–1304. doi: 10.1017/S0033291709991711. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, Huttunen MO, Keshavan MS, Seidman LJ, Tsuang MT. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophrenia Bulletin. 2003;29:653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Archives of General Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- Carletti F, Woolley JB, Bhattacharyya S, Perez-Iglesias R, Poli PF, Valmaggia L, Broome MR, Bramon E, Johns L, Giampietro V, Williams SC, Barker GJ, McGuire PK. Alterations in white matter evident before the onset of psychosis. Schizophrenia Bulletin. 2012;38:1170–1179. doi: 10.1093/schbul/sbs053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. Journal of Abnormal Psychology. 1994;103:171–183. doi: 10.1037//0021-843x.103.2.171. [DOI] [PubMed] [Google Scholar]
- DeRosse P, Nitzburg GC, Kompancaril B, Malhotra AK. The relation between childhood maltreatment and psychosis in patients with schizophrenia and non-psychiatric controls. Schizophrenia Research. 2014;155:66–71. doi: 10.1016/j.schres.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP, Levav I, Shrout PE, Schwartz S, Naveh G, Link BG, Skodol AE, Stueve A. Socioeconomic status and psychiatric disorders: the causation-selection issue. Science. 1992;255:946–952. doi: 10.1126/science.1546291. [DOI] [PubMed] [Google Scholar]
- Eaton WW. Epidemiology of schizophrenia. Epidemiologic reviews. 1985;7:105–126. doi: 10.1093/oxfordjournals.epirev.a036278. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia Research. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Evans GW, Li D, Sepanski Whipple S. Cumulative risk and child development. Psychological Bulletin. 2013;139:1342–1396. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, Ely T, Gutman DA, Ressler KJ. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:2740–2746. doi: 10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychological Medicine. 2003;33:15–21. doi: 10.1017/s0033291702006402. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW Biometrics Research. New York State Psychiatric Institute; New York. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/P) New York, NY: New York State Psychiatric Institute; 2002. [Google Scholar]
- Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H, James AC. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Griffin SL, Mindt MR, Rankin EJ, Ritchie AJ, Scott JG. Estimating premorbid intelligence: comparison of traditional and contemporary methods across the intelligence continuum. Archives of Clinical Neuropsychology. 2002;17:497–507. [PubMed] [Google Scholar]
- Hanssen M, Bak M, Bijl R, Vollebergh W, van Os J. The incidence and outcome of subclinical psychotic experiences in the general population. British Journal of Clinical Psychology. 2005;44:181–191. doi: 10.1348/014466505X29611. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen HU, Van Os J. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. British Medical Journal. 2005;330:11. doi: 10.1136/bmj.38267.664086.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofling AA, Kim JH, Fantz CR, Sands MS, Song SK. Diffusion tensor imaging detects axonal injury and demyelination in the spinal cord and cranial nerves of a murine model of globoid cell leukodystrophy. NMR in Biomedicine. 2009;22:1100–1106. doi: 10.1002/nbm.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social Class and Mental Illness: A Community Study. New York: John Wiley & Sons, Inc.; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Husted JA, Ahmed R, Chow EW, Brzustowicz LM, Bassett AS. Early environmental exposures influence schizophrenia expression even in the presence of strong genetic predisposition. Schizophrenia Research. 2012;137:166–168. doi: 10.1016/j.schres.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jones P, Murray R, Jones P, Rodgers B, Marmot M. Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. The Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biological Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biological Psychiatry. 2009;66:562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Buka SL, Seidman LJ, Goldstein JM, Koren D, Tsuang MT. IQ decline during childhood and adult psychotic symptoms in a community sample: a 19-year longitudinal study. American Journal of Psychiatry. 1998;155:672–677. doi: 10.1176/ajp.155.5.672. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Lyons MJ, Boake C, Xian H, Jacobson KC, Waterman B, Eisen SA, Goldberg J, Faraone SV, Tsuang MT. A discordant twin study of premorbid cognitive ability in schizophrenia. Journal of Clinical and Experimental Neuropsychology. 2006;28:208–224. doi: 10.1080/13803390500360414. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Luck D, Buchy L, Czechowska Y, Bodnar M, Pike GB, Campbell JS, Lepage M. Fronto-temporal disconnectivity and clinical short-term outcome in first episode psychosis: a DTI-tractography study. Journal of Psychiatric Research. 2011;45:369–377. doi: 10.1016/j.jpsychires.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Matheson SL, Shepherd AM, Laurens KR, Carr VJ. A systematic meta-review grading the evidence for non-genetic risk factors and putative antecedents of schizophrenia. Schizophrenia Research. 2011;133:133–142. doi: 10.1016/j.schres.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. The Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria AV, van Zijl PC, Mori S. MRI Atlas of Human White Matter. San Diego: Academic Press; 2010. [Google Scholar]
- Patel S, Mahon K, Wellington R, Zhang J, Chaplin W, Szeszko PR. A meta-analysis of diffusion tensor imaging studies of the corpus callosum in schizophrenia. Schizophrenia Research. 2011;129:149–155. doi: 10.1016/j.schres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? Journal of Psychiatric Research. 2010;44:993–1004. doi: 10.1016/j.jpsychires.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, Brauer J, Asato MR, Khong PL, James AC, Gallego JA, Malhotra AK. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophrenia Bulletin. 2012;38:1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children's self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Archives of General Psychiatry. 2000;57:1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Molecular Psychiatry. 2012;17:1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read J, Os JV, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatrica Scandinavica. 2005;112:330–350. doi: 10.1111/j.1600-0447.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- Reichenberg A. Cognitive impairment as a risk factor for psychosis. Dialogues in Clinical Neuroscience. 2005;7:31–38. doi: 10.31887/DCNS.2005.7.1/areichenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nature Genetics. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Protective factors in children's responses to stress and disadvantage. Annals of the Academy of Medicine, Singapore. 1979;8:324. [PubMed] [Google Scholar]
- Rutter M. Stress, coping, and development: Some issues and some questions. Journal of Child Psychology and Psychiatry. 1981;22:323–356. doi: 10.1111/j.1469-7610.1981.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Medicine. 2005;2:e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Welham J, Chant D, McGrath J. Incidence of schizophrenia does not vary with economic status of the country. Social Psychiatry and Psychiatric Epidemiology. 2006;41:338–340. doi: 10.1007/s00127-006-0041-7. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkinsa KE, Ciccarellid O, Cadera MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Hanssen M, Smirnis NK, Avramopoulos DA, Evdokimidis IK, Stefanis CN, Verdoux H, van Os J. Evidence that three dimensions of psychosis have a distribution in the general population. Psychological Medicine. 2002;32:347–358. doi: 10.1017/s0033291701005141. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, d'Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychological Science. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, De Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. American Journal of Epidemiology. 2002;156:319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, Bentall RP. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective-and cross-sectional cohort studies. Schizophrenia Bulletin. 2012;38:661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JS, Sage CA, Leemans A, Van Hecke W, Callaert D, Peeters R, De Cock P, Lagae L, Sunaert S. Construction of a stereotaxic DTI atlas with full diffusion tensor information for studying white matter maturation from childhood to adolescence using tractography-based segmentations. Human Brain Mapping. 2010;31:470–486. doi: 10.1002/hbm.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welham J, Isohanni M, Jones P, McGrath J. The antecedents of schizophrenia: a review of birth cohort studies. Schizophrenia Bulletin. 2009;35:603–623. doi: 10.1093/schbul/sbn084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Malaspina D, Rabinowitz J. Socioeconomic status at birth is associated with risk of schizophrenia: population-based multilevel study. Schizophrenia Bulletin. 2007;33:1373–1378. doi: 10.1093/schbul/sbm032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Lui S, Liao Y, Du MY, Hu N, Thomas JA, Gong QY. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;45:100–106. doi: 10.1016/j.pnpbp.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. British Medical Journal. 2002;325:1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, Lewis G. A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Archives of General Psychiatry. 2004;61:354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
- Zammit S, Lewis G, Dalman C, Allebeck P. Examining interactions between risk factors for psychosis. The British Journal of Psychiatry. 2010;197(3):207–211. doi: 10.1192/bjp.bp.109.070904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.