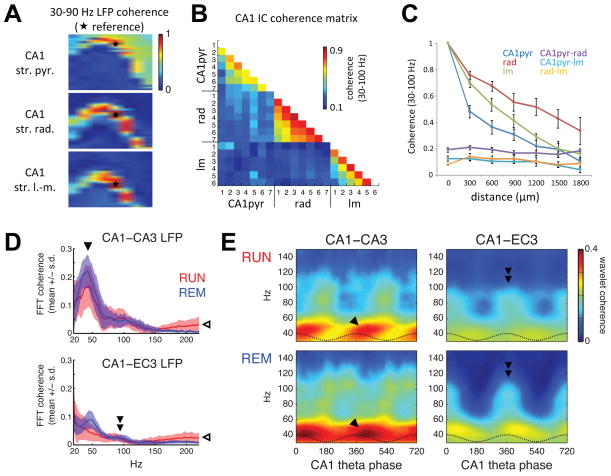
Figure 5. Coherence segregates layer-specific gamma generators.
(A) Gamma (30–90 Hz) coherence maps between LFP recorded from a reference site (white patch with star) and every other recording site on a 256-channel probe spanning most of a transverse plane in the dorsal hippocampus, for CA1 str. pyramidale (top), str. radiatum (middle), and str. lacunosum-moleculare (bottom) references. (B) Gamma coherence between pathway-specific CA1 ICs (extracted separately for each shank). Similar to cross-layer LFP coherence, ICs reflecting different synaptic pathways exhibited low coherence with other CA1 ICs across shanks (numbered 1–7), but high coherence between like ICs from different shanks. (C) Coherence with like ICs decreases monotonically with distance between shanks, whereas coherence between different ICs is low, regardless of shank separation. (D) Mean ± s.e.m. of LFP coherence spectra across sessions for RUN and REM between CA1 and CA3 (top, 3 rats) or CA1 and EC3 (bottom, 3 rats) site pairs from animals used for the single unit analyses. Coherence increased above 100–150 Hz (open arrowhead) due to muscle artifact contamination of LFP recordings (Figure S4). (E) Mean phase-resolved distributions of wavelet phase coherence over theta cycles in the same animals analyzed in (D). Gamma-band coherence between CA1 and CA3 was more theta-modulated during RUN, when CA3 input was stronger (Figure 2E, F) and CA3 gamma oscillations were more theta modulated (Figure 2D). GammaM coherence between CA1 and EC3 was highest (double arrowhead) at the phase of maximal EC3 gamma power (Figure 3) and pyramidal cell spiking (Mizuseki et al., 2009).