Abstract
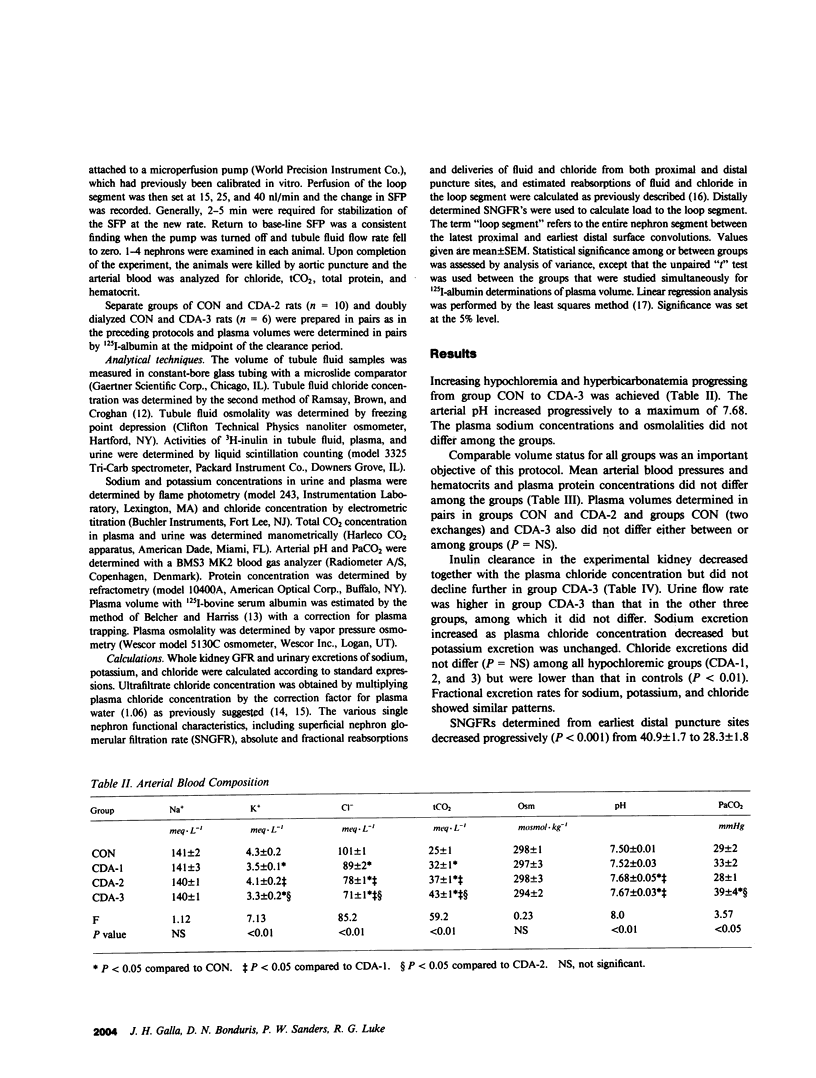
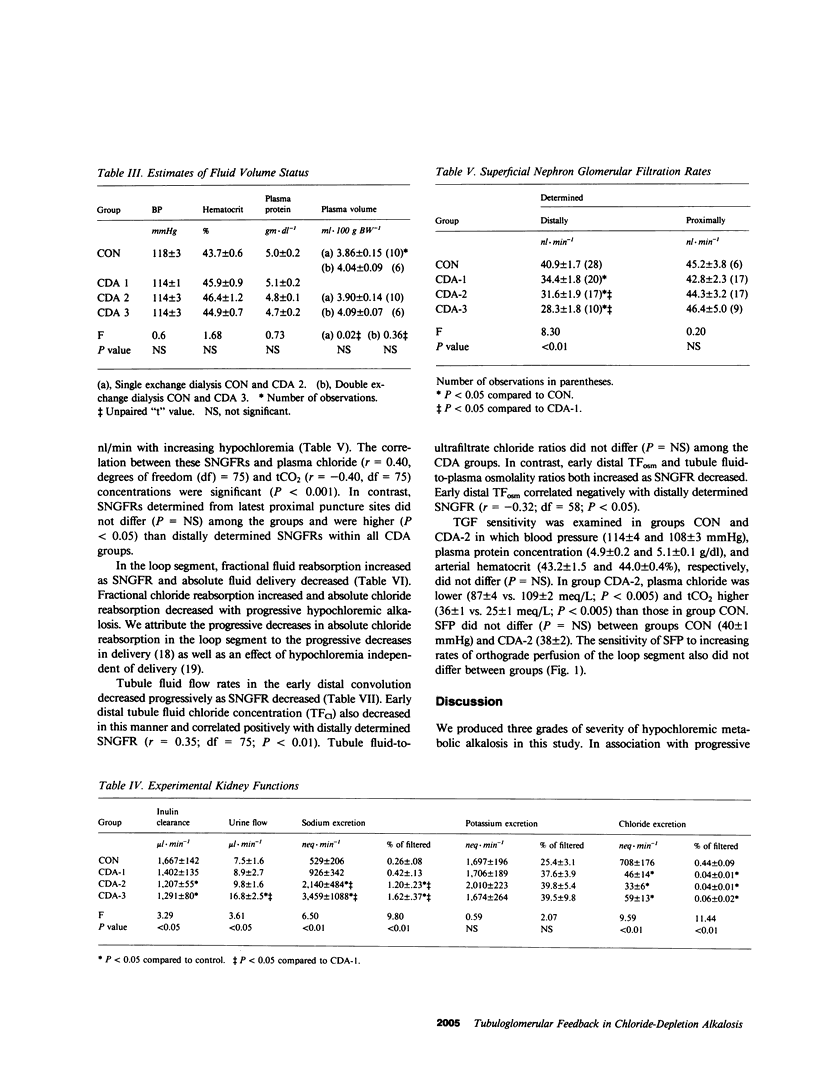
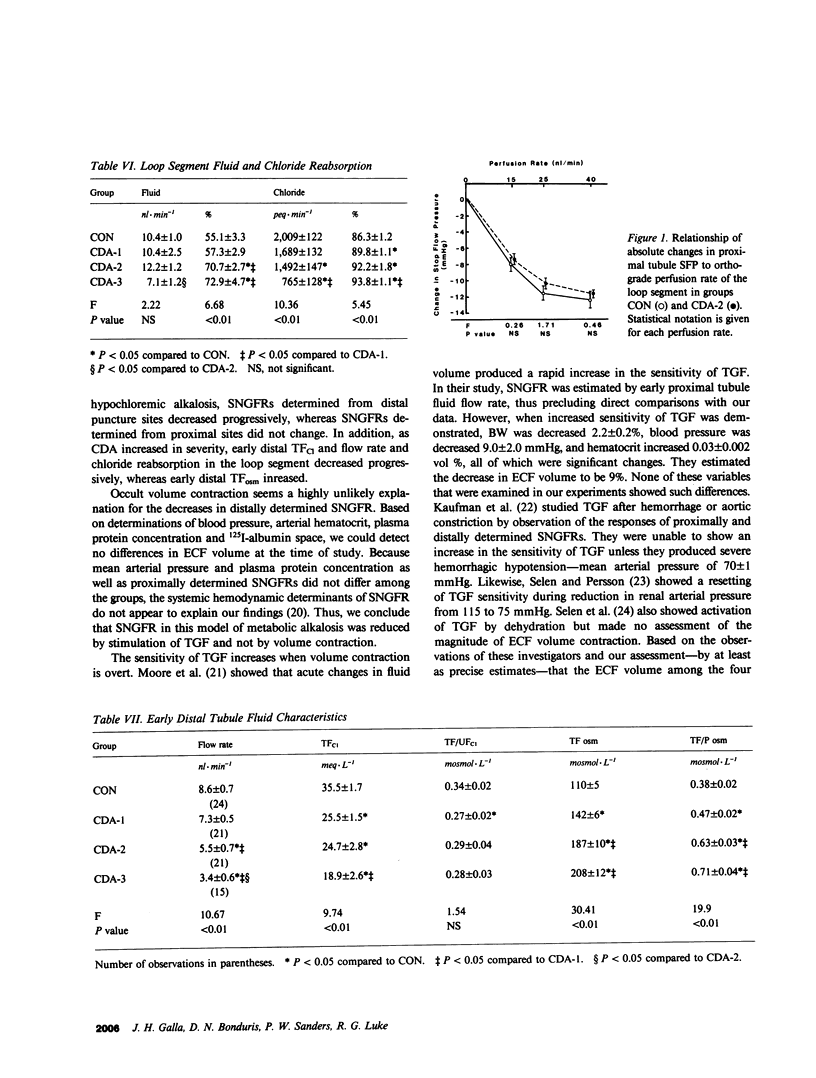
We have recently described reduced superficial nephron glomerular filtration rate (SNGFR) in chloride-depletion alkalosis (CDA) without volume depletion. To elucidate the mechanism of this phenomenon, we studied three degrees of increasing severity of CDA (groups CDA-1, 2, and 3) produced by one or two peritoneal dialyses against 0.15 M NaHCO3 and electrolyte infusions of different Cl and HCO3 content in Sprague-Dawley rats; control rats (CON) were dialyzed against and infused with Ringers-HCO3. Extracellular fluid (ECF) volume was assessed by blood pressure, hematocrit, plasma protein concentration, and 125I-albumin space; none of these variables differed among the four groups. Micropuncture of the latest proximal and earliest distal convolutions was carried out. As CDA intensified from CON to CDA-3 (plasma tCO2 25 +/- 1 to 43 +/- 1 meq/L; P less than 0.01), distally determined SNGFR declined progressively (40.9 +/- 1.7 to 28.3 +/- 1.8 nl/min; P less than 0.01), while in early distal tubule fluid, flow rate (8.6 +/- 0.7 to 3.4 +/- 0.6 nl/min) and Cl concentration (36 +/- 2 to 19 +/- 3 meq/L) decreased and osmolality (110 +/- 5 to 208 +/- 12 mosmol/kg) increased (P less than 0.01), and, in the loop segment, Cl reabsorption decreased progressively (2,009 +/- 112 to 765 +/- 128 peq/min; P less than 0.01). In early distal tubule fluid, Cl concentration correlated positively and osmolality negatively with distally determined SNGFR (P less than 0.05). Proximally determined SNGFRs did not differ among the four groups. Proximal tubule stop-flow pressure responses to increasing rates of orthograde perfusion of the loop segment from 0 to 40 nl/min did not differ between groups CON and CDA-2. We interpret these data to show that reductions in SNGFR in CDA in the rat can occur by tubuloglomerular feedback (TGF) in the absence of differences in ECF volume or of alterations in TGF sensitivity during metabolic alkalosis. Of the proposed signals for TGF sensed by the macula densa, distal tubule fluid osmolality or some related variable is the signal most compatible with our data.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELCHER E. H., HARRISS E. B. Studies of plasma volume, red cell volume and total blood volume in young growing rats. J Physiol. 1957 Nov 14;139(1):64–78. doi: 10.1113/jphysiol.1957.sp005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P. D., Navar L. G. Relationship between tubulo-glomerular feedback responses and perfusate hypotonicity. Kidney Int. 1982 Sep;22(3):234–239. doi: 10.1038/ki.1982.160. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Konnen K. S. Relation of distal tubular delivery and reabsorptive rate to nephron filtration. Am J Physiol. 1977 Oct;233(4):F315–F324. doi: 10.1152/ajprenal.1977.233.4.F315. [DOI] [PubMed] [Google Scholar]
- Booker B. B., Williams R. H., Luke R. G. Effect of volume expansion and plasma chloride on function of the loop segment. Am J Physiol. 1983 Jul;245(1):F41–F47. doi: 10.1152/ajprenal.1983.245.1.F41. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Biagi B., Giebisch G. Control mechanisms of bicarbonate transport across the rat proximal convoluted tubule. Am J Physiol. 1982 May;242(5):F532–F543. doi: 10.1152/ajprenal.1982.242.5.F532. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Liu F. Y. Metabolic alkalosis in the rat. Evidence that reduced glomerular filtration rather than enhanced tubular bicarbonate reabsorption is responsible for maintaining the alkalotic state. J Clin Invest. 1983 May;71(5):1141–1160. doi: 10.1172/JCI110864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello Aires M., Malnic G. Renal handling of sodium and potassium during hypochloremic alkalosis in the rat. Pflugers Arch. 1972;331(3):215–225. doi: 10.1007/BF00589128. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Seldin D. W., Kokko J. P. Segmental chloride reabsorption in the rat nephron as a function of load. Am J Physiol. 1978 Feb;234(2):F97–105. doi: 10.1152/ajprenal.1978.234.2.F97. [DOI] [PubMed] [Google Scholar]
- Galla J. H., Bonduris D. N., Dumbauld S. L., Luke R. G. Segmental chloride and fluid handling during correction of chloride-depletion alkalosis without volume expansion in the rat. J Clin Invest. 1984 Jan;73(1):96–106. doi: 10.1172/JCI111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla J. H., Bonduris D. N., Luke R. G. Correction of acute chloride-depletion alkalosis in the rat without volume expansion. Am J Physiol. 1983 Feb;244(2):F217–F221. doi: 10.1152/ajprenal.1983.244.2.F217. [DOI] [PubMed] [Google Scholar]
- Galla J. H., Kirchner K. A., Kotchen T. A., Luke R. G. Effect of hypochloremia on loop segment chloride and solute reabsorption in the rat during volume expansion. Kidney Int. 1981 Nov;20(5):569–574. doi: 10.1038/ki.1981.178. [DOI] [PubMed] [Google Scholar]
- Gamble J. L., Ross S. G. THE FACTORS IN THE DEHYDRATION FOLLOWING PYLORIC OBSTRUCTION. J Clin Invest. 1925 Jun;1(5):403–423. doi: 10.1172/JCI100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsche H. U., Múller-Suur R., Hegel U., Hierholzer K. Electrical conductivity of tubular fluid of the rat nephron. Micropuncture study of the diluting segment in situ. Pflugers Arch. 1980 Jan;383(2):113–121. doi: 10.1007/BF00581871. [DOI] [PubMed] [Google Scholar]
- Harris C. A., Baer P. G., Chirito E., Dirks J. H. Composition of mammalian glomerular filtrate. Am J Physiol. 1974 Oct;227(4):972–976. doi: 10.1152/ajplegacy.1974.227.4.972. [DOI] [PubMed] [Google Scholar]
- Kaufman J. S., Hamburger R. J., Flamenbaum W. Tubuloglomerular feedback response after hypotensive hemorrhage. Ren Physiol. 1982;5(4):173–181. doi: 10.1159/000172854. [DOI] [PubMed] [Google Scholar]
- Kunau R. T., Jr, Frick A., Rector F. C., Jr, Seldin D. W. Micropuncture study of the proximal tubular factors responsible for the maintenance of alkalosis during potassium deficiency in the rat. Clin Sci. 1968 Apr;34(2):223–231. [PubMed] [Google Scholar]
- Maddox D. A., Gennari F. J. Proximal tubular bicarbonate reabsorption and PCO2 in chronic metabolic alkalosis in the rat. J Clin Invest. 1983 Oct;72(4):1385–1395. doi: 10.1172/JCI111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Suur R., Ulfendahl H. R., Persson A. E. Evidence for tubuloglomerular feedback in juxtamedullary nephrons of young rats. Am J Physiol. 1983 Apr;244(4):F425–F431. doi: 10.1152/ajprenal.1983.244.4.F425. [DOI] [PubMed] [Google Scholar]
- Ploth D. W., Rudulph J., LaGrange R., Navar L. G. Tubuloglomerular feedback and single nephron function after converting enzyme inhibition in the rat. J Clin Invest. 1979 Nov;64(5):1325–1335. doi: 10.1172/JCI109589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnermann J., Briggs J. Concentration-dependent sodium chloride transport as the signal in feedback control of glomerular filtration rate. Kidney Int Suppl. 1982 Aug;12:S82–S89. [PubMed] [Google Scholar]
- Schnermann J., Briggs J., Schubert G. In situ studies of the distal convoluted tubule in the rat. I. Evidence for NaCl secretion. Am J Physiol. 1982 Aug;243(2):F160–F166. doi: 10.1152/ajprenal.1982.243.2.F160. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Ploth D. W., Hermle M. Activation of tubulo-glomerular feedback by chloride transport. Pflugers Arch. 1976 Apr 6;362(3):229–240. doi: 10.1007/BF00581175. [DOI] [PubMed] [Google Scholar]
- Selén G., Müller-Suur R., Persson A. E. Activation of the tubuloglomerular feedback mechanism in dehydrated rats. Acta Physiol Scand. 1983 Jan;117(1):83–89. doi: 10.1111/j.1748-1716.1983.tb07181.x. [DOI] [PubMed] [Google Scholar]
- Selén G., Persson A. E. Effects of reduced renal artery pressure on feedback control of glomerular filtration. Am J Physiol. 1983 Mar;244(3):F342–F348. doi: 10.1152/ajprenal.1983.244.3.F342. [DOI] [PubMed] [Google Scholar]
- Wright F. S., Briggs J. P. Feedback control of glomerular blood flow, pressure, and filtration rate. Physiol Rev. 1979 Oct;59(4):958–1006. doi: 10.1152/physrev.1979.59.4.958. [DOI] [PubMed] [Google Scholar]


