Abstract
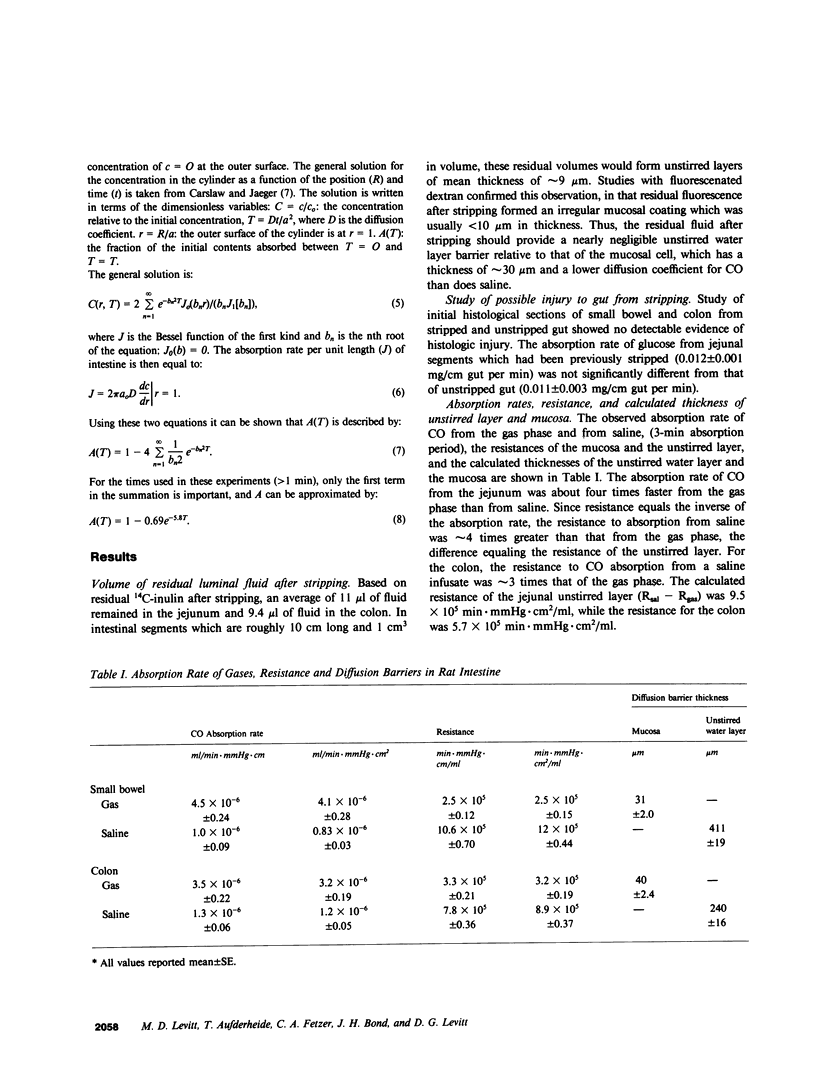
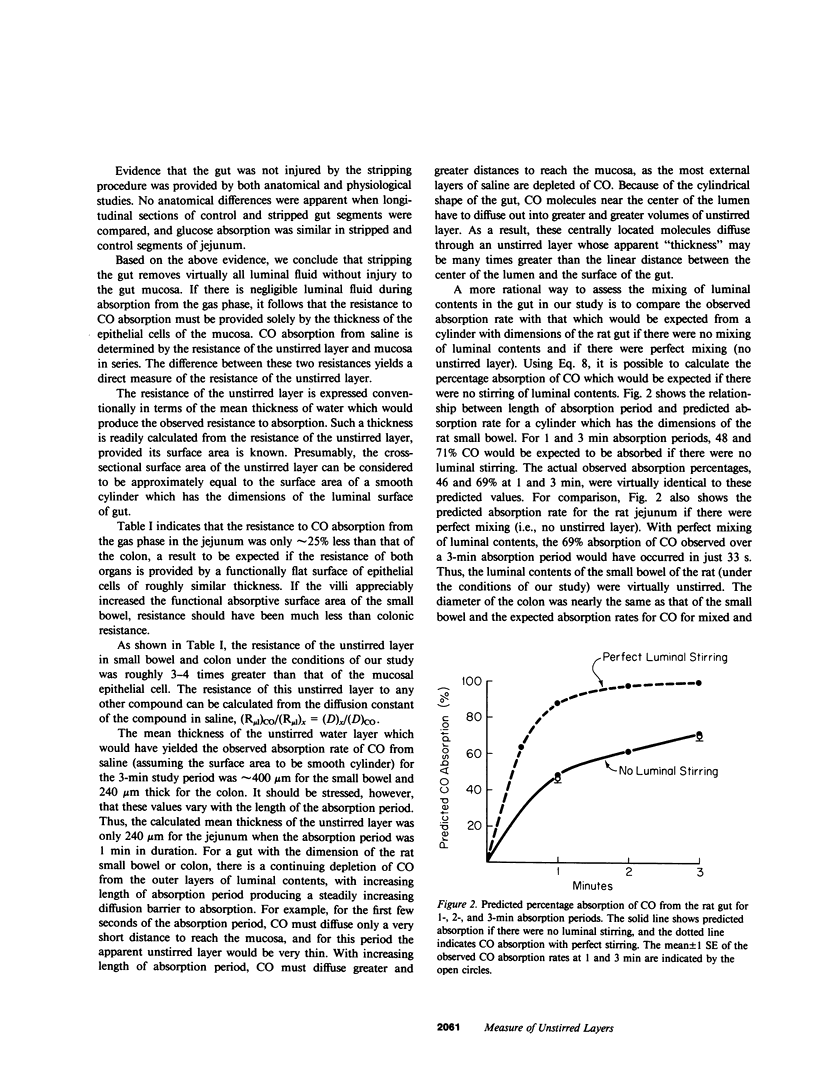
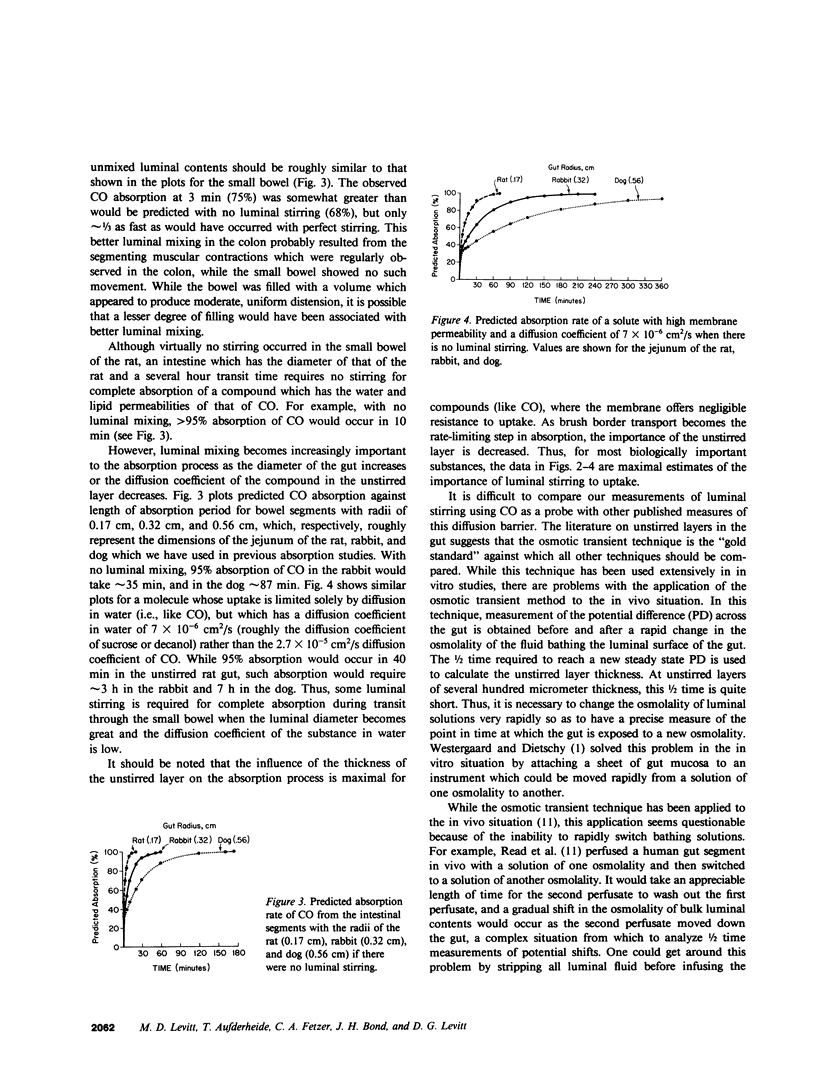
We used carbon monoxide (CO) as a probe to quantitatively measure intestinal unstirred water layers in vivo. CO has several features that make it uniquely well suited to measure the unstirred layer in that its tight binding to hemoglobin makes uptake diffusion limited, and its relatively high lipid solubility renders membrane resistance negligible relative to the water barriers of the unstirred layer and epithelial cell. The unique application of CO was the measurement of the absorption rate of CO both from the gas phase as well as a solute dissolved in saline. Several lines of evidence showed that a gut stripped free of saline and then filled with gas contained a negligible unstirred layer. Thus, absorption of CO from the gas phase measured resistance of just the epithelial cell. Subtraction of this value from the resistance of CO absorption from saline provided a direct measure of unstirred layer resistance. Studies in the rat showed for a 3-min absorption period that the conventionally calculated apparent unstirred layer for the jejunum was 411 micron and for the colon was 240 micron. However, this conventionally calculated unstirred layer resistance did not truly depict the situation in the rat gut, since there was a continuing depletion of CO from outer surfaces of luminal contents throughout the experiment period. This produced a continually increasing diffusion barrier with time. Calculation of expected absorption rate from unstirred cylinders with the dimensions of the rat gut indicated that there was virtually no stirring in the small intestine and minimal stirring in the colon. The technique described in this paper appears to be simpler and to require fewer assumptions for validity than other techniques previously used to measure unstirred layers in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond J. H., Levitt D. G., Levitt M. D. Quantitation of countercurrent exchange during passive absorption from the dog small intestine: evidence for marked species differences in the efficiency of exchange. J Clin Invest. 1977 Feb;59(2):308–318. doi: 10.1172/JCI108642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone J. A. Diffusion barrier in the small intestine. Science. 1983 Apr 8;220(4593):221–222. doi: 10.1126/science.6828892. [DOI] [PubMed] [Google Scholar]
- Diamond J. M. A rapid method for determining voltage-concentration relations across membranes. J Physiol. 1966 Mar;183(1):83–100. doi: 10.1113/jphysiol.1966.sp007852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley V. H., Kutchai H. The effect of the red cell membrane and a diffusion boundary layer on the rate of oxygen uptake by human erythrocytes. J Physiol. 1981 Jul;316:75–83. doi: 10.1113/jphysiol.1981.sp013773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampp M., Lundgren O., Nilsson N. J. Extravascular shunting of oxygen in the small intestine of the cat. Acta Physiol Scand. 1968 Apr;72(4):396–403. doi: 10.1111/j.1748-1716.1968.tb03864.x. [DOI] [PubMed] [Google Scholar]
- Krogh A. The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J Physiol. 1919 May 20;52(6):391–408. doi: 10.1113/jphysiol.1919.sp001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read N. W., Barber D. C., Levin R. J., Holdsworth C. D. Unstirred layer and kinetics of electrogenic glucose absorption in the human jejunum in situ. Gut. 1977 Nov;18(11):865–876. doi: 10.1136/gut.18.11.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson K. W., Millar D. B., Jacobs L. R., Gray G. M. Intestinal diffusion barrier: unstirred water layer or membrane surface mucous coat? Science. 1981 Dec 11;214(4526):1241–1244. doi: 10.1126/science.7302593. [DOI] [PubMed] [Google Scholar]
- Thomson A. B., Dietschy J. M. Experimental demonstration of the effect of the unstirred water layer on the kinetic constants of the membrane transport of D-glucose in rabbit jejunum. J Membr Biol. 1980 Jun 15;54(3):221–229. doi: 10.1007/BF01870238. [DOI] [PubMed] [Google Scholar]
- Westergaard H., Dietschy J. M. Delineation of the dimensions and permeability characteristics of the two major diffusion barriers to passive mucosal uptake in the rabbit intestine. J Clin Invest. 1974 Sep;54(3):718–732. doi: 10.1172/JCI107810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. A., Dietschy J. M. Characterization of bile acid absorption across the unstirred water layer and brush border of the rat jejunum. J Clin Invest. 1972 Dec;51(12):3015–3025. doi: 10.1172/JCI107129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winne D. Dependence of intestinal absorption in vivo on the unstirred layer. Naunyn Schmiedebergs Arch Pharmacol. 1978 Sep;304(2):175–181. doi: 10.1007/BF00495554. [DOI] [PubMed] [Google Scholar]


