Abstract
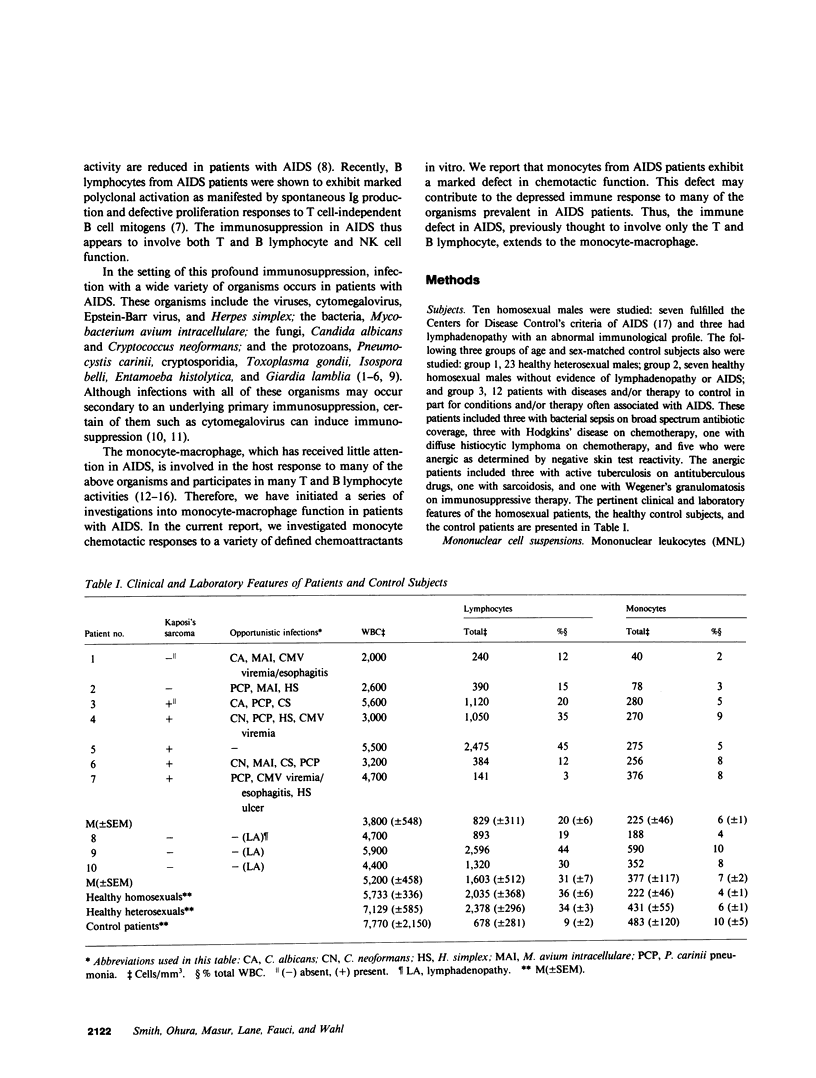
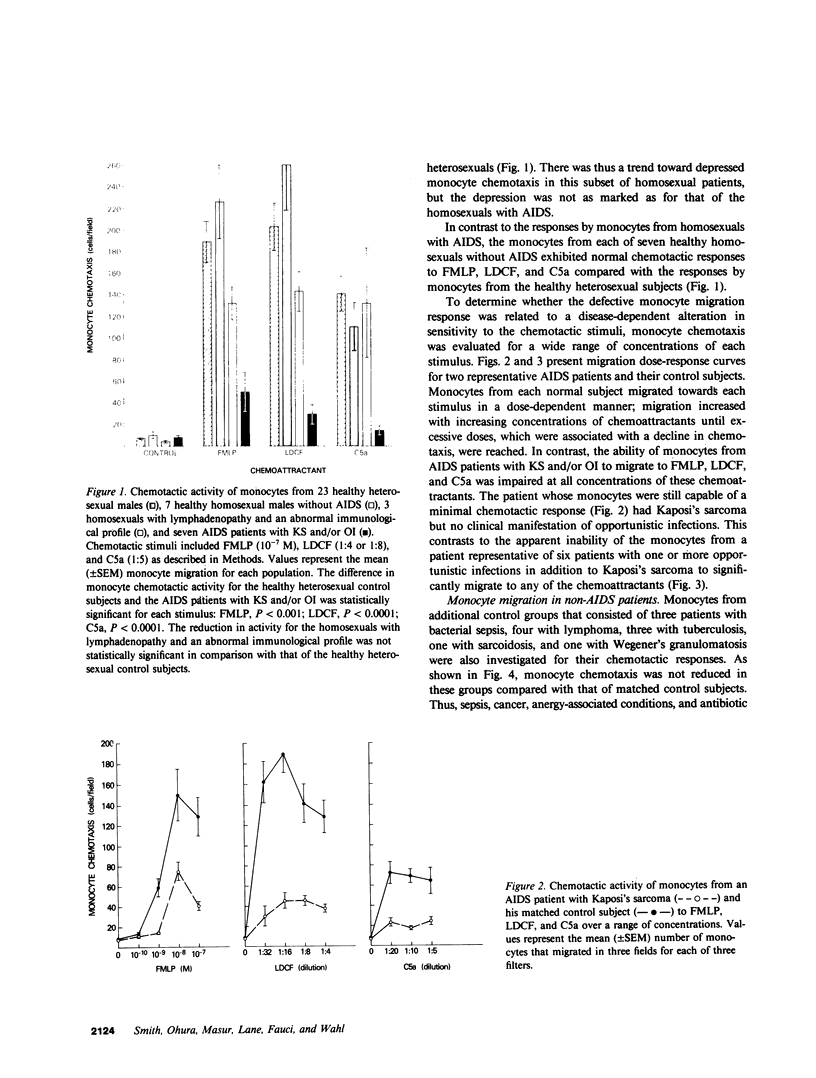
The ineffective immune response in patients with the acquired immune deficiency syndrome (AIDS) contributes to severe and widespread infections and unrestricted growth by certain tumors. To determine whether monocyte dysfunction contributes to this immunosuppressed condition, we investigated monocyte chemotaxis in patients with AIDS. Using three different chemotactic stimuli, N-formylmethionylleucylphenylalanine, lymphocyte-derived chemotactic factor, and C5a des Arg, we studied the chemotactic responses of monocytes from seven homosexual men with AIDS, three homosexuals with lymphadenopathy and an abnormal immunological profile, seven healthy homosexual men, and 23 heterosexual control individuals. Monocytes from each of the AIDS patients with Kaposi's sarcoma and/or opportunistic infection exhibited a marked reduction in chemotaxis to all stimuli compared with the healthy control subjects. The reduced chemotactic responses were observed over a wide range of concentrations for each stimulus. Monocytes from AIDS patients who had clinically apparent opportunistic infection(s) exhibited a greater reduction in monocyte migration to all three stimuli than monocytes from the AIDS patient with only Kaposi's sarcoma. Monocytes from each of three homosexuals with lymphadenopathy and an abnormal immunological profile exhibited decreased chemotactic responses that were intermediate between those of the AIDS patients and the healthy heterosexual control subjects. In contrast to these findings, monocytes from each of seven healthy homosexuals exhibited normal chemotactic responses to the same stimuli. In addition, monocytes from AIDS patients exhibited reduced chemotaxis to soluble products of Giardia lamblia, one of several protozoan parasites prevalent in AIDS patients. Thus the immune abnormality in AIDS, previously thought to involve only the T-, B-, and natural killer lymphocytes, extends to the monocyte-macrophage. Defective monocyte migratory function may contribute to the depressed inflammatory response to certain organisms and to the apparent unrestricted growth of certain neoplasms in patients with AIDS.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman L. C., Snyderman R., Oppenheim J. J., Mergenhagen S. E. A human mononuclear leukocyte chemotactic factor: characterization, specificity and kinetics of production by homologous leukocytes. J Immunol. 1973 Mar;110(3):801–810. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cianciolo G. J., Lostrom M. E., Tam M., Snyderman R. Murine malignant cells synthesize a 19,000-dalton protein that is physicochemically and antigenically related to the immunosuppressive retroviral protein, P15E. J Exp Med. 1983 Sep 1;158(3):885–900. doi: 10.1084/jem.158.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciolo G. J., Matthews T. J., Bolognesi D. P., Snyderman R. Macrophage accumulation in mice is inhibited by low molecular weight products from murine leukemia viruses. J Immunol. 1980 Jun;124(6):2900–2905. [PubMed] [Google Scholar]
- Cianciolo G. J., Phipps D., Snyderman R. Human malignant and mitogen-transformed cells contain retroviral P15E-related antigen. J Exp Med. 1984 Mar 1;159(3):964–969. doi: 10.1084/jem.159.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Current W. L., Reese N. C., Ernst J. V., Bailey W. S., Heyman M. B., Weinstein W. M. Human cryptosporidiosis in immunocompetent and immunodeficient persons. Studies of an outbreak and experimental transmission. N Engl J Med. 1983 May 26;308(21):1252–1257. doi: 10.1056/NEJM198305263082102. [DOI] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Friedman-Kien A. E., Laubenstein L. J., Rubinstein P., Buimovici-Klein E., Marmor M., Stahl R., Spigland I., Kim K. S., Zolla-Pazner S. Disseminated Kaposi's sarcoma in homosexual men. Ann Intern Med. 1982 Jun;96(6 Pt 1):693–700. doi: 10.7326/0003-4819-96-6-693. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. S., Schroff R., Schanker H. M., Weisman J. D., Fan P. T., Wolf R. A., Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981 Dec 10;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Greene J. B., Sidhu G. S., Lewin S., Levine J. F., Masur H., Simberkoff M. S., Nicholas P., Good R. C., Zolla-Pazner S. B., Pollock A. A. Mycobacterium avium-intracellulare: a cause of disseminated life-threatening infection in homosexuals and drug abusers. Ann Intern Med. 1982 Oct;97(4):539–546. doi: 10.7326/0003-4819-97-4-539. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Levy M. H., Wheelock E. F. The role of macrophages in defense against neoplastic disease. Adv Cancer Res. 1974;20:131–163. doi: 10.1016/s0065-230x(08)60110-4. [DOI] [PubMed] [Google Scholar]
- Masur H., Michelis M. A., Greene J. B., Onorato I., Stouwe R. A., Holzman R. S., Wormser G., Brettman L., Lange M., Murray H. W. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med. 1981 Dec 10;305(24):1431–1438. doi: 10.1056/NEJM198112103052402. [DOI] [PubMed] [Google Scholar]
- Mildvan D., Mathur U., Enlow R. W., Romain P. L., Winchester R. J., Colp C., Singman H., Adelsberg B. R., Spigland I. Opportunistic infections and immune deficiency in homosexual men. Ann Intern Med. 1982 Jun;96(6 Pt 1):700–704. doi: 10.7326/0003-4819-96-6-700. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Vadas M. A., Whitelaw A., Gamble J. Role of major histocompatibility complex gene products in delayed-type hypersensitivity. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2486–2490. doi: 10.1073/pnas.73.7.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Howard R. J. Effect of virus infections on the function of the immune system. Annu Rev Microbiol. 1970;24:525–538. doi: 10.1146/annurev.mi.24.100170.002521. [DOI] [PubMed] [Google Scholar]
- Pape J. W., Liautaud B., Thomas F., Mathurin J. R., St Amand M. M., Boncy M., Pean V., Pamphile M., Laroche A. C., Johnson W. D., Jr Characteristics of the acquired immunodeficiency syndrome (AIDS) in Haiti. N Engl J Med. 1983 Oct 20;309(16):945–950. doi: 10.1056/NEJM198310203091603. [DOI] [PubMed] [Google Scholar]
- Pierce C. W., Kapp J. A., Benacerraf B. Regulation by the H-2 gene complex of macrophage-lymphoid cell interactions in secondary antibody responses in vitro. J Exp Med. 1976 Aug 1;144(2):371–381. doi: 10.1084/jem.144.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Snyderman R. Depression of macrophage function by a factor produced by neoplasms: a merchanism for abrogation of immune surveillance. J Immunol. 1976 Oct;117(4):1243–1249. [PubMed] [Google Scholar]
- Pinching A. J., McManus T. J., Jeffries D. J., Moshtael O., Donaghy M., Parkin J. M., Munday P. E., Harris J. R. Studies of cellular immunity in male homosexuals in London. Lancet. 1983 Jul 16;2(8342):126–130. doi: 10.1016/s0140-6736(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Pitchenik A. E., Fischl M. A., Dickinson G. M., Becker D. M., Fournier A. M., O'Connell M. T., Colton R. M., Spira T. J. Opportunistic infections and Kaposi's sarcoma among Haitians: evidence of a new acquired immunodeficiency state. Ann Intern Med. 1983 Mar;98(3):277–284. doi: 10.7326/0003-4819-98-3-277. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Carney W. P., Richter B. S., Black P. H., Hirsch M. S. Mechanisms of immunosuppression in cytomegaloviral mononucleosis. J Infect Dis. 1980 Apr;141(4):488–495. doi: 10.1093/infdis/141.4.488. [DOI] [PubMed] [Google Scholar]
- Rook A. H., Masur H., Lane H. C., Frederick W., Kasahara T., Macher A. M., Djeu J. Y., Manischewitz J. F., Jackson L., Fauci A. S. Interleukin-2 enhances the depressed natural killer and cytomegalovirus-specific cytotoxic activities of lymphocytes from patients with the acquired immune deficiency syndrome. J Clin Invest. 1983 Jul;72(1):398–403. doi: 10.1172/JCI110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach J., Popovic M., Gilden R. V., Gonda M. A., Sarngadharan M. G., Gallo R. C. Serological analysis of a subgroup of human T-lymphotropic retroviruses (HTLV-III) associated with AIDS. Science. 1984 May 4;224(4648):503–505. doi: 10.1126/science.6200937. [DOI] [PubMed] [Google Scholar]
- Shin H. S., Hayden M., Langley S., Kaliss N., Smith M. R. Antibody-mediated suppression of grafted lymphoma. III. Evaluation of the role of thymic function, non-thymus-derived lymphocytes, macrophages, platelets, and polymorphonuclear leukocytes in syngeneic and allogeneic hosts. J Immunol. 1975 Apr;114(4):1255–1263. [PubMed] [Google Scholar]
- Shin H. S., Snyderman R., Friedman E., Mellors A., Mayer M. M. Chemotactic and anaphylatoxic fragment cleaved from the fifth component of guinea pig complement. Science. 1968 Oct 18;162(3851):361–363. doi: 10.1126/science.162.3851.361. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Lopez C., Hammer G. S., Brown A. E., Kornfeld S. J., Gold J., Hassett J., Hirschman S. Z., Cunningham-Rundles C., Adelsberg B. R. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981 Dec 10;305(24):1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- Smith P. D., Elson C. O., Keister D. B., Nash T. E. Human host response to Giardia lamblia. I. Spontaneous killing by mononuclear leukocytes in vitro. J Immunol. 1982 Mar;128(3):1372–1376. [PubMed] [Google Scholar]
- Smith P. D., Gillin F. D., Kaushal N. A., Nash T. E. Antigenic analysis of Giardia lamblia from Afghanistan, Puerto Rico, Ecuador, and Oregon. Infect Immun. 1982 May;36(2):714–719. doi: 10.1128/iai.36.2.714-719.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. D., Gillin F. D., Spira W. M., Nash T. E. Chronic giardiasis: studies on drug sensitivity, toxin production, and host immune response. Gastroenterology. 1982 Oct;83(4):797–803. [PubMed] [Google Scholar]
- Smith P. D., Keister D. B., Elson C. O. Human host response to Giardia lamblia. II. Antibody-dependent killing in vitro. Cell Immunol. 1983 Dec;82(2):308–315. doi: 10.1016/0008-8749(83)90164-8. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wilton J. M., Rosenstreich D. L., Oppenheim J. J. The role of macrophages in the production of lymphokines by T and B lymphocytes. J Immunol. 1975 Apr;114(4):1296–1301. [PubMed] [Google Scholar]
- Weinberger O., Herrmann S. H., Mescher M. F., Benacerraf B., Burakoff S. J. Cellular interactions in the generation of cytolytic T lymphocyte responses: role of Ia-positive splenic adherent cells in presentation in H-2 antigen. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6091–6095. doi: 10.1073/pnas.77.10.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]