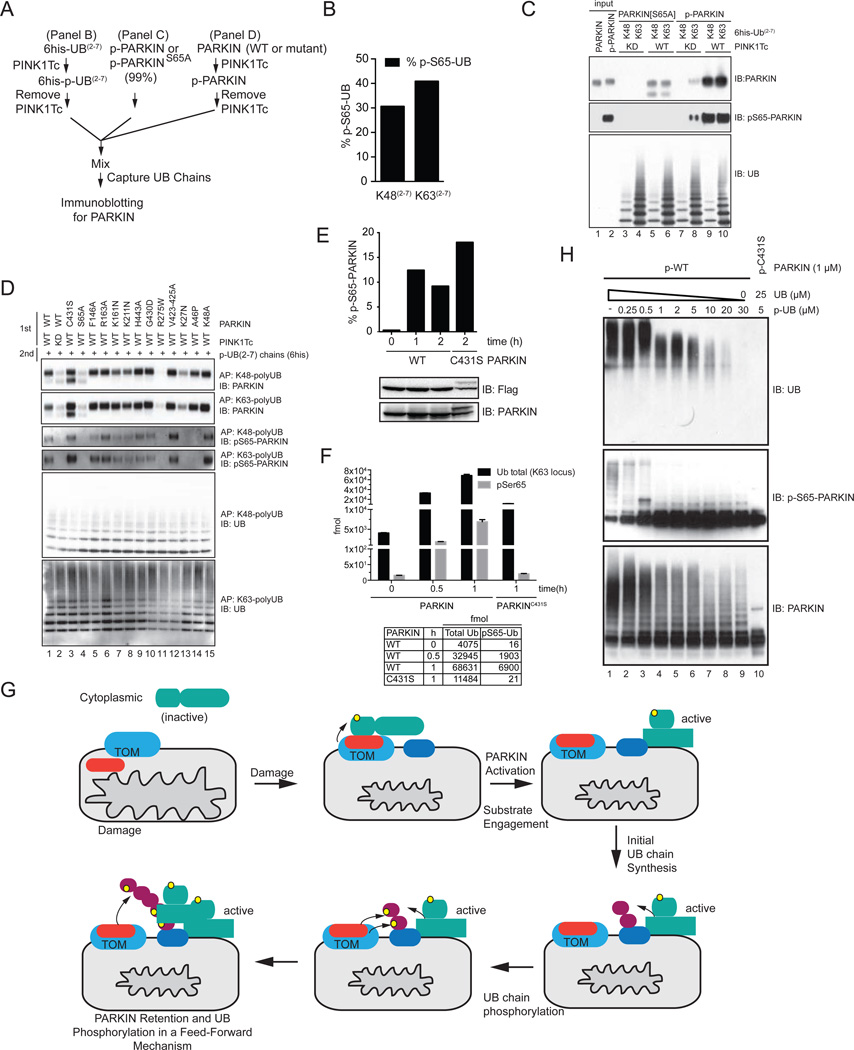
Figure 7. PINK1-dependent phosphorylation of PARKIN’s UBL domain promotes association with PINK1-phosphorylated poly-UB chains.
(A) Scheme for examining phosphorylation-dependent binding of PARKIN to UB chains.
(B) Stoichiometry of PINK1Tc-dependent phosphorylation of K48 and K63 UB(2–7) chains used in panel C and D measured by AQUA proteomics.
(C) Phosphorylation dependent binding of PARKIN to phosphorylated UB chains. p-WT and p-S65A PARKIN were incubated with the indicated poly-UB chains as shown in panel A and UB chains captured with Ni-NTA beads prior to immunoblotting. The p-WT PARKIN used in this experiment was 99% phosphorylated on S65 as determined by AQUA (Figure S5C).
(D) Binding of a panel of PD patient and structure-based mutants of PARKIN to poly-p-UB chains. PARKIN proteins were phosphorylated with PINK1Tc, the PINK1Tc removed, and the proteins incubated with K48 and K63 UB(2–7) chains previously phosphorylated with PINK1Tc and the PINK1Tc removed (panel A). UB chains were captured and association with PARKIN examined by immunoblotting.
(E) Phosphorylation of WT PARKIN and PARKINC431S on S65 in response to depolarization was measured by AQUA proteomics.
(F) Absolute levels of UB and p-S65 UB on mitochondria from HeLa Flp-In T-REx cells expressing WT PARKIN or PARKINC431S, in the presence or absence of depolarization measured by AQUA proteomics (derived from primary data in Figure 1K).
(G) A feed forward model for PARKIN activation and retention on the mitochondrial surface in response to depolarization. See text for details.
(H) Effect of p-UB (99% phosphorylated) on UB chain synthesis by p-WT PARKIN (99% phosphorylated). The total UB concentration was 30 µM.