Abstract
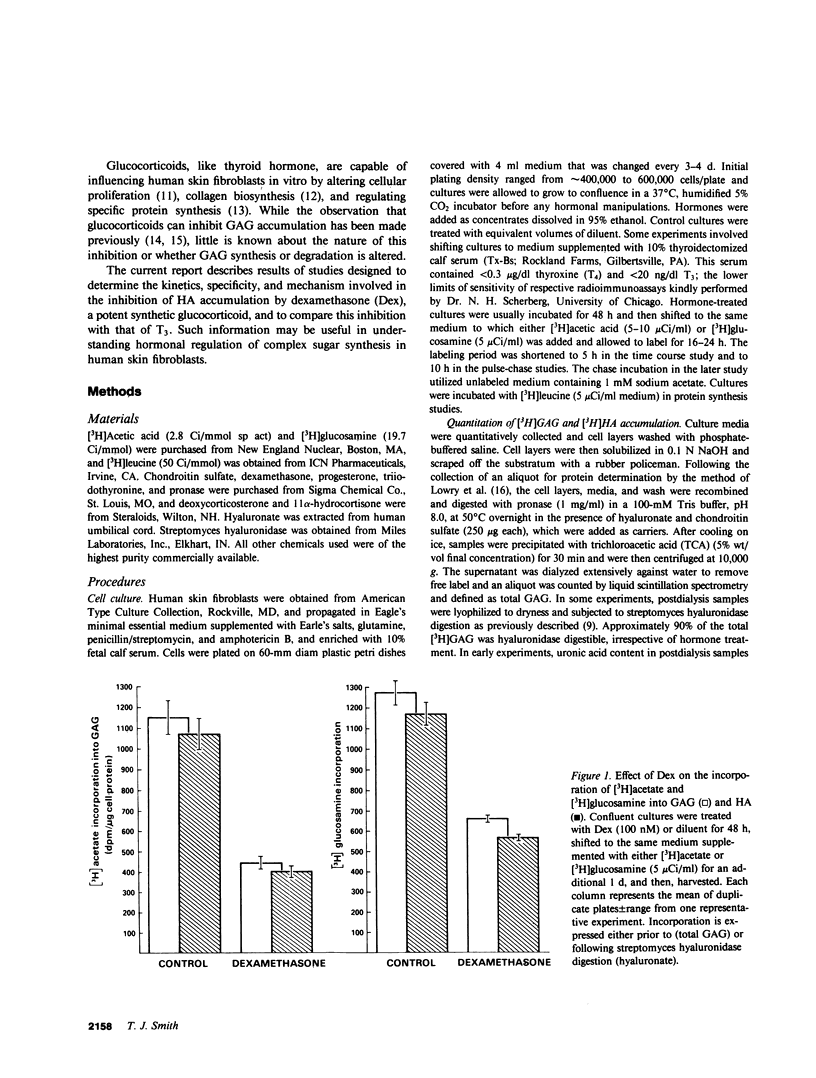
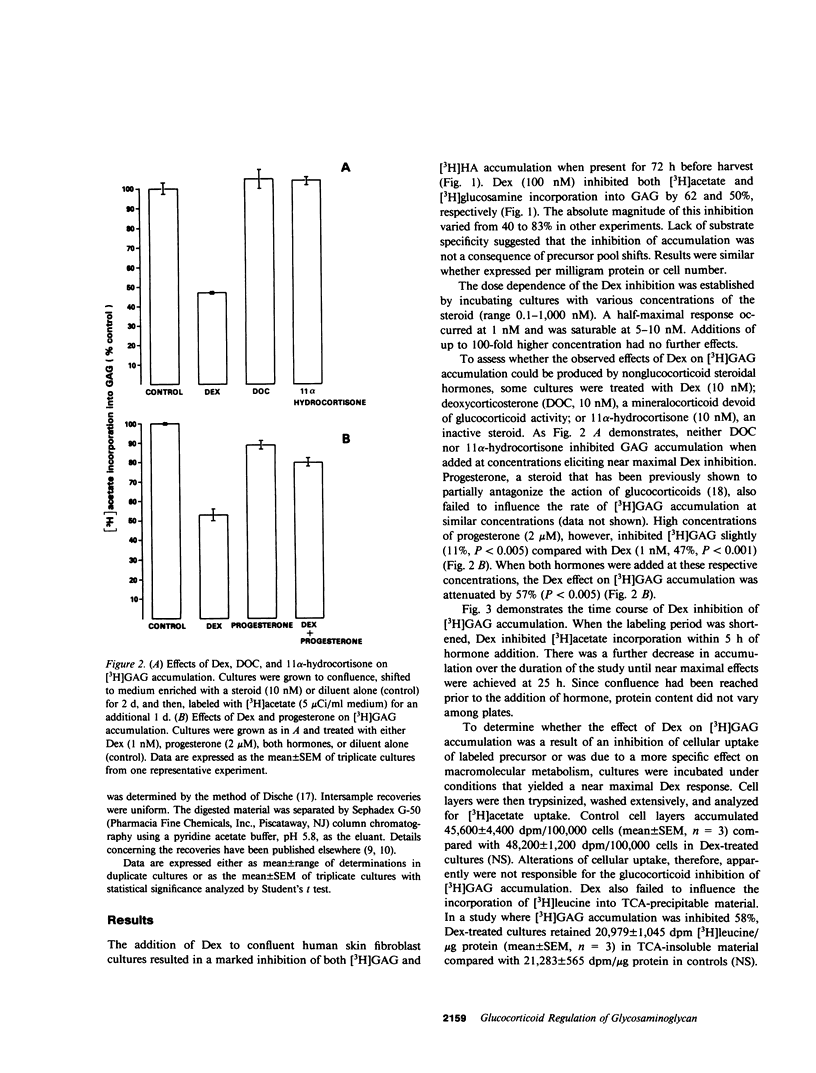
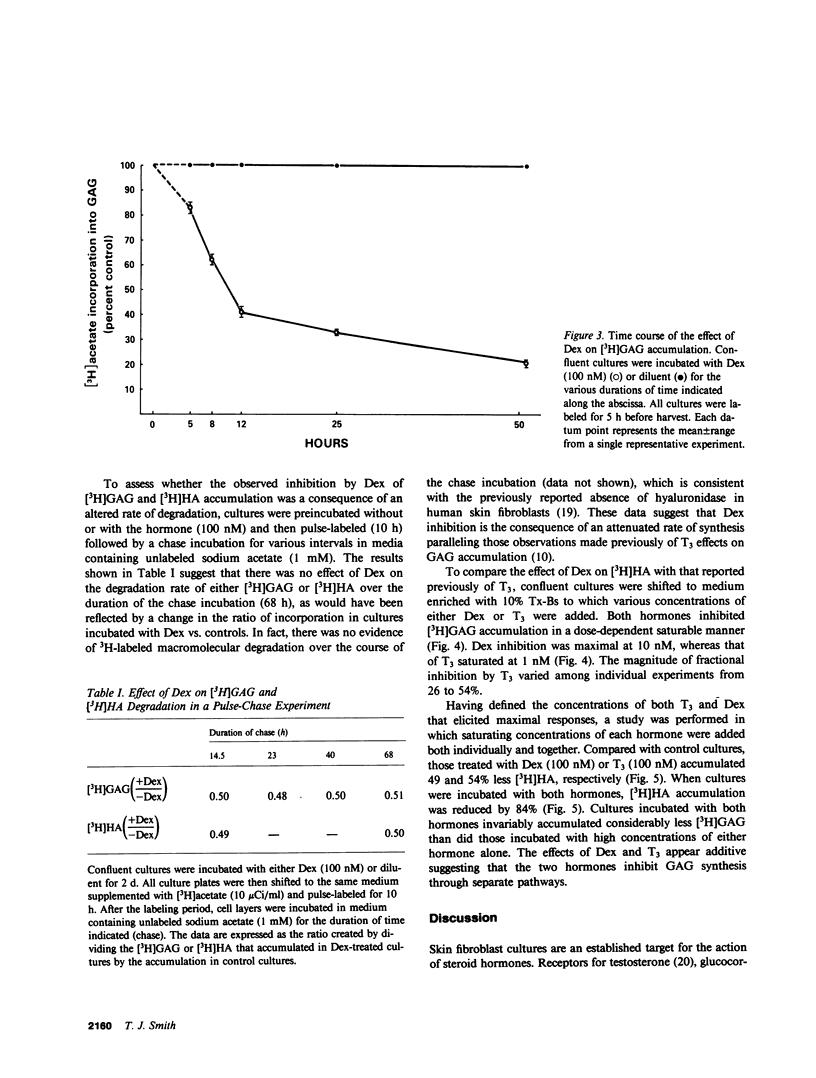
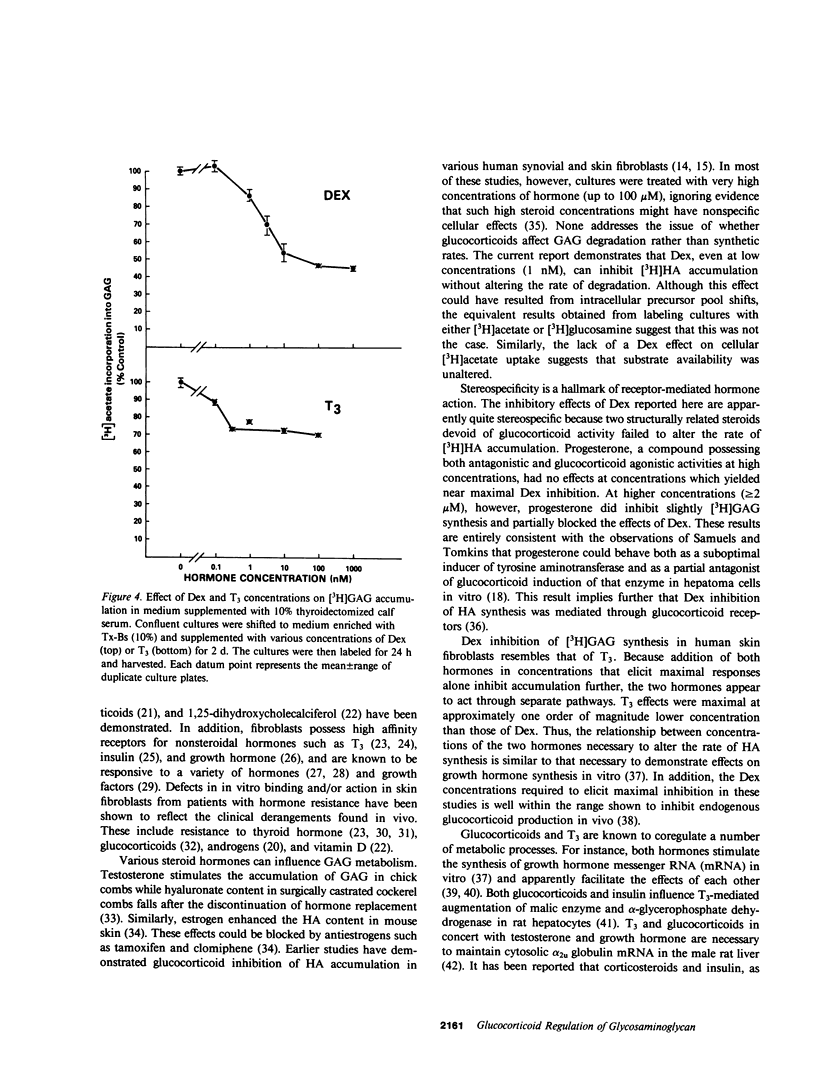
The effects of dexamethasone on glycosaminoglycan accumulation were examined in confluent human skin fibroblasts in vitro. The glucocorticoid consistently inhibited the incorporation of either [3H]acetate or [3H]glucosamine into hyaluronate when added to culture medium 72 h before harvest. This effect was half-maximal at approximately 1 nM and maximal at 5-10 nM. Inhibition occurred within 5 h of hormone addition and was near maximal by 25 h. 11 alpha-hydrocortisone (10 nM), deoxycorticosterone (10 nM), and progesterone (100 nM) failed to inhibit this accumulation; however, progesterone (2 microM), a known glucocorticoid antagonist at high concentration, could attenuate the response to dexamethasone by 57%. Cultures were pulse-labeled and then chase incubated for up to 68 h. No difference in the rate of [3H]hyaluronate degradation could be demonstrated in steroid-treated cultures. Triiodothyronine (T3) can also inhibit synthesis of hyaluronate in fibroblasts (Smith, T. J., Y. Murata, A. L. Horwitz, L. Philipson, and S. Refetoff, 1982, J. Clin. Invest., 70:1066-1073). Both T3 and dexamethasone could inhibit glycosaminoglycan accumulation in a dose-dependent manner. Maximal T3 effects were achieved at 1 nM and those of dexamethasone at 10 nM. Saturating concentrations of T3 and dexamethasone added alone inhibited [3H]hyaluronate by 54 and 49%, respectively. When both hormones were added, accumulation was inhibited by 84%. Dexamethasone inhibits [3H]hyaluronate accumulation in a time, dose-dependent, and stereo-specific manner. The rate of glycosaminoglycan degradation was unaffected, and thus, the steroid inhibited the rate of macromolecular synthesis. This effect was likely mediated through glucocorticoid receptors. Hyaluronate synthesis in skin fibroblasts appears to be regulated by both glucocorticoids and T3 through different pathways.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbogast B., Hopwood J. J., Dorfman A. Absence of hyaluronidase in cultured human skin fibroblasts. Biochem Biophys Res Commun. 1975 Nov 3;67(1):376–382. doi: 10.1016/0006-291x(75)90326-5. [DOI] [PubMed] [Google Scholar]
- BEIERWALTES W. H., BOLLET A. J. Mucopolysaccharide content of skin in patients with pretibial myxedema. J Clin Invest. 1959 Jun;38(6):945–948. doi: 10.1172/JCI103877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson G. S., Dalferes E. R., Jr Urinary excretion of mucopolysaccharides in normal individuals and in the Marfan syndrome. Biochim Biophys Acta. 1965 Jul 1;101(2):183–192. doi: 10.1016/0926-6534(65)90049-7. [DOI] [PubMed] [Google Scholar]
- Bernal J., Refetoff S., DeGroot L. J. Abnormalities of triiodothyronine binding to lymphocyte and fibroblast nuclei from a patient with peripheral tissue resistance to thyroid hormone action. J Clin Endocrinol Metab. 1978 Dec;47(6):1266–1272. doi: 10.1210/jcem-47-6-1266. [DOI] [PubMed] [Google Scholar]
- Bruning P. F., Meyer W. J., 3rd, Migeon C. J. Glucocorticoid receptor in cultured human skin fibroblasts. J Steroid Biochem. 1979 Jun;10(6):587–593. doi: 10.1016/0022-4731(79)90510-7. [DOI] [PubMed] [Google Scholar]
- Chait A., Kanter R., Green W., Kenny M. Defective thyroid hormone action in fibroblasts cultured from subjects with the syndrome of resistance to thyroid hormones. J Clin Endocrinol Metab. 1982 Apr;54(4):767–772. doi: 10.1210/jcem-54-4-767. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P., Vingerhoeds A., Brandon D., Eil C., Pugeat M., DeVroede M., Loriaux D. L., Lipsett M. B. Primary cortisol resistance in man. A glucocorticoid receptor-mediated disease. J Clin Invest. 1982 Jun;69(6):1261–1269. doi: 10.1172/JCI110565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. W., Huang L. S., Larner J. Insulin stimulation of glycogen synthase activity in cultured human fibroblasts from diabetic and control subjects. Diabetologia. 1980;18(2):109–113. doi: 10.1007/BF00290485. [DOI] [PubMed] [Google Scholar]
- Eil C., Fein H. G., Smith T. J., Furlanetto R. W., Bourgeois M., Stelling M. W., Weintraub B. D. Nuclear binding of [125I]triiodothyronine in dispersed cultured skin fibroblasts from patients with resistance to thyroid hormone. J Clin Endocrinol Metab. 1982 Sep;55(3):502–510. doi: 10.1210/jcem-55-3-502. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Edelman I. S. Glucocorticoid receptors in rat kidney: the binding of tritiated-dexamethasone. Endocrinology. 1973 Apr;92(4):1005–1013. doi: 10.1210/endo-92-4-1005. [DOI] [PubMed] [Google Scholar]
- GABRILOVE J. L., LUDWIG A. W. The histogenesis of myxedema. J Clin Endocrinol Metab. 1957 Aug;17(8):925–932. doi: 10.1210/jcem-17-8-925. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Jen P., Freychet P. Insulin receptors in human circulating cells and fibroblasts. Proc Natl Acad Sci U S A. 1972 Mar;69(3):747–751. doi: 10.1073/pnas.69.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Goldstein S., Posner B. I., Guyda H. J. Insulin-like peptides stimulate metabolism but not proliferation of human fibroblasts. Am J Physiol. 1980 Aug;239(2):E125–E131. doi: 10.1152/ajpendo.1980.239.2.E125. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Dorfman A. Glycosaminoglycan synthesis by cultured human skin fibroblasts after transformation with simian virus 40. J Biol Chem. 1977 Jul 25;252(14):4777–4785. [PubMed] [Google Scholar]
- Ivarie R. D., Baxter J. D., Morris J. A. Interaction of thyroid and glucocorticoid hormones in rat pituitary tumor cells. Specificity and diversity of the responses analyzed by two-dimensional gel electrophoresis. J Biol Chem. 1981 May 10;256(9):4520–4528. [PubMed] [Google Scholar]
- Keenan B. S., Meyer W. J., 3rd, Hadjian A. J., Jones H. W., Migeon C. J. Syndrome of androgen insensitivity in man: absence of 5 alpha-dihydrotestosterone binding protein in skin fibroblasts. J Clin Endocrinol Metab. 1974 Jun;38(6):1143–1146. doi: 10.1210/jcem-38-6-1143. [DOI] [PubMed] [Google Scholar]
- Khalid B. A., Gyorki S., Warne G. L., Funder J. W. Cystic fibrosis and normal fibroblasts have identical glucocorticoid receptor profiles and induced protein responses. Clin Endocrinol (Oxf) 1983 Apr;18(4):407–415. doi: 10.1111/j.1365-2265.1983.tb00586.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamberg S. I., Dorfman A. Synthesis and degradation of hyaluronic acid in the cultured fibroblasts of Marfan's disease. J Clin Invest. 1973 Oct;52(10):2428–2433. doi: 10.1172/JCI107433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman U. A., Eil C., Marx S. J. Resistance to 1,25-dihydroxyvitamin D. Association with heterogeneous defects in cultured skin fibroblasts. J Clin Invest. 1983 Feb;71(2):192–200. doi: 10.1172/JCI110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapleson J. L., Buchwald M. Effect of cycloheximide and dexamethasone phosphate on hyaluronic acid synthesis and secretion in cultured human skin fibroblasts. J Cell Physiol. 1981 Nov;109(2):215–222. doi: 10.1002/jcp.1041090204. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martial J. A., Seeburg P. H., Guenzi D., Goodman H. M., Baxter J. D. Regulation of growth hormone gene expression: synergistic effects of thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4293–4295. doi: 10.1073/pnas.74.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby J. C., Dale S. L. Comparison of absorption, disposal, and activity of soluble and repository corticosteroid esters. Clin Pharmacol Ther. 1969 May-Jun;10(3):344–349. doi: 10.1002/cpt1969103344. [DOI] [PubMed] [Google Scholar]
- Murata Y., Refetoff S., Horwitz A. L., Smith T. J. Hormonal regulation of glycosaminoglycan accumulation in fibroblasts from patients with resistance to thyroid hormone. J Clin Endocrinol Metab. 1983 Dec;57(6):1233–1239. doi: 10.1210/jcem-57-6-1233. [DOI] [PubMed] [Google Scholar]
- Murphy L. J., Vrhovsek E., Lazarus L. Identification and characterization of specific growth hormone receptors in cultured human fibroblasts. J Clin Endocrinol Metab. 1983 Dec;57(6):1117–1124. doi: 10.1210/jcem-57-6-1117. [DOI] [PubMed] [Google Scholar]
- Nelson D. H. Corticosteroid-induced changes in phospholipid membranes as mediators of their action. Endocr Rev. 1980 Spring;1(2):180–199. doi: 10.1210/edrv-1-2-180. [DOI] [PubMed] [Google Scholar]
- Ponec M., Hasper I., Vianden G. D., Bachra B. N. Effects of glucocorticosteroids on primary human skin fibroblasts. II. Effects on total protein and collagen biosynthesis by confluent cell cultures. Arch Dermatol Res. 1977 Aug 22;259(2):125–134. doi: 10.1007/BF00557952. [DOI] [PubMed] [Google Scholar]
- Ponec M., de Haas C., Bachra B. N., Polano M. K. Effects of glucocorticosteroids on primary human skin fibroblasts. I. Inhibition of the proliferation of cultured primary human skin and mouse L929 fibroblasts. Arch Dermatol Res. 1977 Aug 22;259(2):117–123. doi: 10.1007/BF00557951. [DOI] [PubMed] [Google Scholar]
- SCHILLER S., BENDITT E. P., DORFMAN A. Effect of testosterone and cortisone on the hexosamine content and metachromasia of chick combs. Endocrinology. 1952 May;50(5):504–510. doi: 10.1210/endo-50-5-504. [DOI] [PubMed] [Google Scholar]
- Saarni H., Tammi M. Time and concentration dependence of the action of cortisol on fibroblasts in vitro. Biochim Biophys Acta. 1978 Apr 19;540(1):117–126. doi: 10.1016/0304-4165(78)90440-3. [DOI] [PubMed] [Google Scholar]
- Saarni H., Tammi M., Vuorio E. Effects of cortisol on glycosaminoglycans synthesized by normal and rheumatoid synovial fibroblasts in vitro. Scand J Rheumatol. 1977;6(4):222–224. doi: 10.3109/03009747709095454. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Control of growth hormone synthesis in cultured GH1 cells by 3,5,3'-triiodo-L-thyronine and glucocorticoid agonists and antagonists: studies on the independent and synergistic regulation of the growth hormone response. Biochemistry. 1979 Feb 20;18(4):715–721. doi: 10.1021/bi00571a025. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tomkins G. M. Relation of steroid structure to enzyme induction in hepatoma tissue culture cells. J Mol Biol. 1970 Aug 28;52(1):57–74. doi: 10.1016/0022-2836(70)90177-4. [DOI] [PubMed] [Google Scholar]
- Sassa S., Kappas A. Induction of aminolevulinate synthase and porphyrins in cultured liver cells maintained in chemically defined medium. Permissive effects of hormones on induction process. J Biol Chem. 1977 Apr 10;252(7):2428–2436. [PubMed] [Google Scholar]
- Smith T. J., Horwitz A. L., Refetoff S. The effect of thyroid hormone on glycosaminoglycan accumulation in human skin fibroblasts. Endocrinology. 1981 Jun;108(6):2397–2399. doi: 10.1210/endo-108-6-2397. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Murata Y., Horwitz A. L., Philipson L., Refetoff S. Regulation of glycosaminoglycan synthesis by thyroid hormone in vitro. J Clin Invest. 1982 Nov;70(5):1066–1073. doi: 10.1172/JCI110694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turakainen H., Larjava H., Saarni H., Penttinen R. Synthesis of hyaluronic acid and collagen in skin fibroblasts cultured from patients with osteogenesis imperfecta. Biochim Biophys Acta. 1980 Apr 3;628(4):388–397. doi: 10.1016/0304-4165(80)90388-8. [DOI] [PubMed] [Google Scholar]
- Uzuka M., Nakajima K., Ohta S., Mori Y. The mechanism of estrogen-induced increase in hyaluronic acid biosynthesis, with special reference to estrogen receptor in the mouse skin. Biochim Biophys Acta. 1980 Jan 17;627(2):199–206. doi: 10.1016/0304-4165(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Wilson E. J., McMurray W. C. Insulin and cortisol increase the response of rat hepatocytes in primary culture to 3,3',5 triiodothyronine. Biochem Biophys Res Commun. 1980 Mar 13;93(1):179–185. doi: 10.1016/s0006-291x(80)80263-4. [DOI] [PubMed] [Google Scholar]


