Figure 1. Potential glycosylation on mouse Notch1.
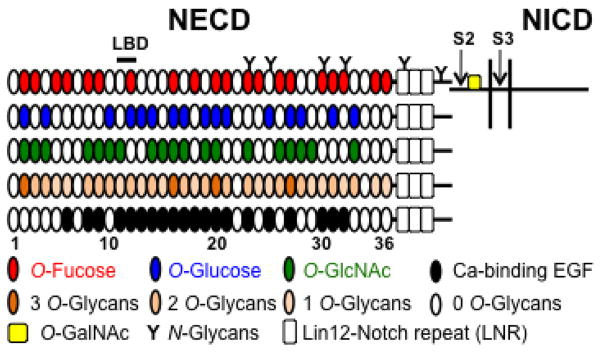
Mouse Notch1 is a heterodimer where the NECD is bound non-covalently to the transmembrane-intracellular domain (shown for the top diagram, not drawn to scale). The positions of the S2 and S3 protease cleavage sites are shown. The NICD is released upon cleavage at S3. The NECD of mouse Notch1 consists of 36 EGF repeats (ovals) and 3 Lin12-Notch repeats (LNR, open rectangles). EGF repeats 11–12 are the ligand binding domain (LBD). EGF repeats containing the consensus sequence for O-Fucose (red), O-Glucose (blue), or O-GlcNAc (green) are shown. EGF repeats with any of these O-glycans are shown in different colors according to whether they have one, two or three potential O-glycans modifications. Ca-binding EGF repeats are shown in black. O-GalNAc glycans may exist near the ADAM10/17-cleavage site 2 (S2) and thereby regulate a subsequent cleavage by γ-secretase complex at S3 within the transmembrane.
