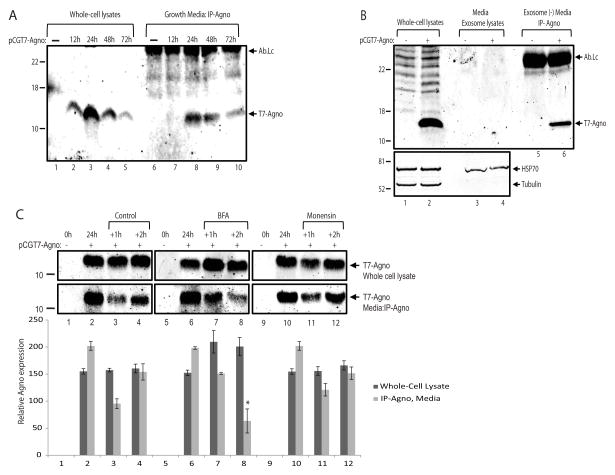
Figure 3. Agnoprotein release may involve conventional pathway.
A. Immunoprecipitation of agnoprotein in growth media from T98-G cells. Cells were transfected with an expression polasmid encoding T7-tagged agnoprotein (pCGT7-Agno) and growth media was collected at 0, 12, 24, 48, and 72h post-transfections simultaneously along with whole cell extracts. Agnoprotein was immunoprecipitated from the growth media, and analyzed by western blotting (lanes 6 – 10). Whole cell protein extracts were also processed and loaded in parallel for the cellular expression of agnoprotein (lanes 1 – 5). Ab-Lc points the light chain of T7 tag antibody. B. The secreted agnoprotein is not associated with extracellular vesicles. Extracellular vesicles were isolated from growth media of either control cells or cells expressing agnoprotein by ExoQuick exosome precipitation solution (System Biosciences, SBI) according to the manufacturer’s instructions. The precipitated exosomes were lysed with TNN buffer containing 1% NP40, and analyzed by Western blot for the detection of agnoprotein (upper panel, lanes 3 and 4) and Hsp70 (bottom panel, lanes 3 and 4) proteins. The exosome depleted growth media was subjected to immunoprecipitation for agnoprotein (lanes 5 and 6) by using an anti-T7 tag monoclonal antibody. Whole cell protein extracts were also processed in parallel for the cellular expression of agnoprotein (lanes 1 and 2). C. Agnoprotein secretion is suppressed by Brefeldin A. T98G cells were transfected with pCGT7-Agno expression plasmid and grouped as 24h, +1h, and +2h. At 24h post-transfection, growth media and whole cell protein extracts were obtained from the first 24h group for the cellular and secreted expression of agnoprotein. Meanwhile, the growth media of +1h and +2h groups were aspirated, cells were washed twice with PBS, and treated with Brefeldin A (10 μg/ml) or Monensin (1 μM) in fresh growth media. At 1h and 2h post-treatment, growth medium was collected and processed for immunoprecipitation of agnoprotein (lower panel). In parallel, whole cell protein extracts were also prepared and analyzed by Western blot for its cellular expression (top panel). Bar graph represents the relative expression of agnoprotein in whole cell lysates and growth medium presented in the upper panels. All experiments were carried out in triplicate.