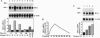Abstract
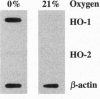
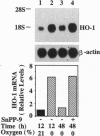
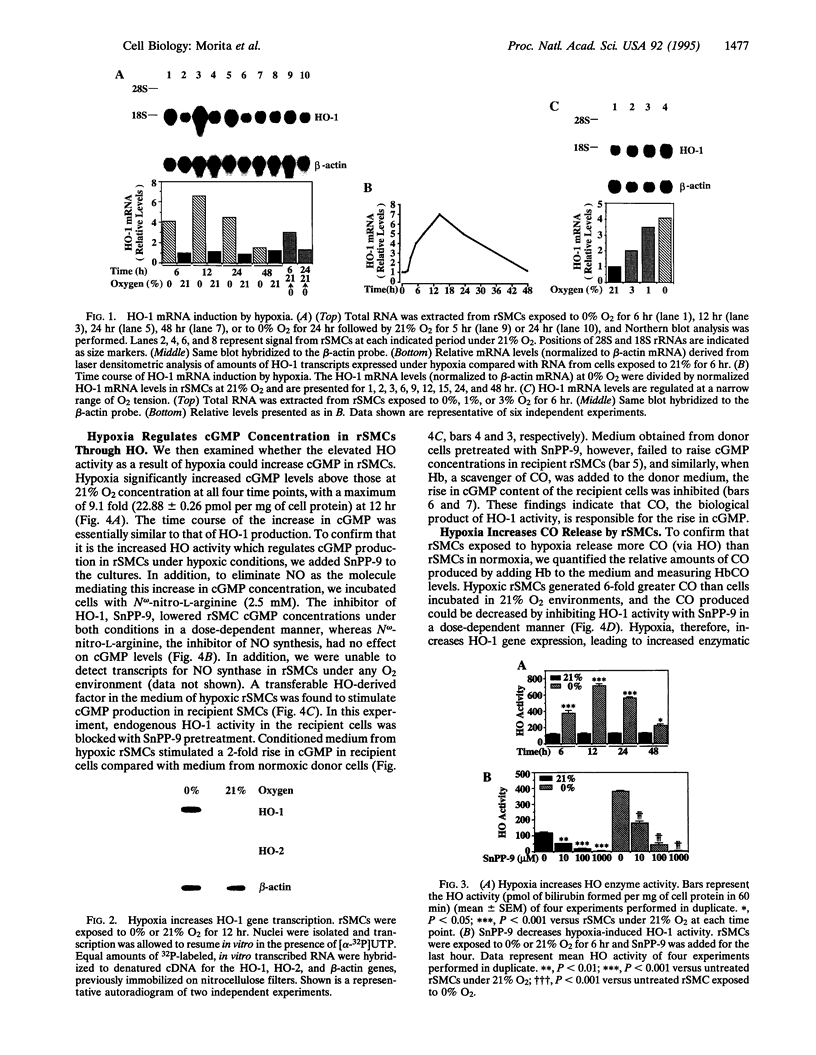
Carbon monoxide (CO) is a product of the enzyme heme oxygenase (HO; EC 1.14.99.3). In vascular smooth muscle cells, exogenously administered CO increases cyclic guanosine 3',5'-monophosphate (cGMP), which is an important regulator of vessel tone. We report here that smooth muscle cells produce CO via HO and that it regulates cGMP levels in these cells. Hypoxia, which has profound effects on vessel tone, significantly increased the transcriptional rate of the HO-1 gene resulting in corresponding increases of its mRNA and HO enzymatic activity. In addition, under the same conditions, rat aortic and pulmonary artery smooth muscle cells accumulated high levels of cGMP following a similar time course to that of HO-1 production. The increased accumulation of cGMP in smooth muscle cells required the enzymatic activity of HO, since it was abolished by a specific HO inhibitor, tin protoporphyrin. In contrast, N omega-nitro-L-arginine, a potent inhibitor of nitric oxide (NO) synthesis, had no effect on cGMP produced by smooth muscle cells, indicating that NO is not responsible for the activation of guanylyl cyclase in this setting. Furthermore, conditioned medium from hypoxic smooth muscle cells stimulated cGMP production in recipient cells and this stimulation was completely inhibited by tin protoporphyrin or hemoglobin, an inhibitor of CO production and a scavenger of CO, respectively. This report shows that HO-1 is expressed by vascular smooth muscle cells and that its product, CO, may regulate vascular tone under physiologic and pathophysiologic (such as hypoxic) conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam J., Shibahara S., Smith A. Transcriptional activation of the heme oxygenase gene by heme and cadmium in mouse hepatoma cells. J Biol Chem. 1989 Apr 15;264(11):6371–6375. [PubMed] [Google Scholar]
- Balla G., Jacob H. S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J. W., Vercellotti G. M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992 Sep 5;267(25):18148–18153. [PubMed] [Google Scholar]
- Brüne B., Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987 Oct;32(4):497–504. [PubMed] [Google Scholar]
- Goldberg M. A., Schneider T. J. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994 Feb 11;269(6):4355–4359. [PubMed] [Google Scholar]
- Gunther S., Alexander R. W., Atkinson W. J., Gimbrone M. A., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982 Feb;92(2):289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh W. M., Harsh G. R., 4th, Starksen N. F., Rocco C. M., Williams L. T. Transcriptional regulation of the A and B chain genes of platelet-derived growth factor in microvascular endothelial cells. J Biol Chem. 1988 Jun 15;263(17):8470–8472. [PubMed] [Google Scholar]
- Kourembanas S., Hannan R. L., Faller D. V. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J Clin Invest. 1990 Aug;86(2):670–674. doi: 10.1172/JCI114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S., Marsden P. A., McQuillan L. P., Faller D. V. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991 Sep;88(3):1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S., McQuillan L. P., Leung G. K., Faller D. V. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest. 1993 Jul;92(1):99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. E., Temizer D. H., Clifford J. A., Quertermous T. Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J Biol Chem. 1991 Aug 25;266(24):16188–16192. [PubMed] [Google Scholar]
- Maines M. D., Ibrahim N. G., Kappas A. Solubilization and partial purification of heme oxygenase from rat liver. J Biol Chem. 1977 Aug 25;252(16):5900–5903. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Maines M. D., Trakshel G. M., Kutty R. K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986 Jan 5;261(1):411–419. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- McQuillan L. P., Leung G. K., Marsden P. A., Kostyk S. K., Kourembanas S. Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am J Physiol. 1994 Nov;267(5 Pt 2):H1921–H1927. doi: 10.1152/ajpheart.1994.267.5.H1921. [DOI] [PubMed] [Google Scholar]
- Ramos K. S., Lin H., McGrath J. J. Modulation of cyclic guanosine monophosphate levels in cultured aortic smooth muscle cells by carbon monoxide. Biochem Pharmacol. 1989 Apr 15;38(8):1368–1370. doi: 10.1016/0006-2952(89)90347-x. [DOI] [PubMed] [Google Scholar]
- Rodgers P. A., Vreman H. J., Dennery P. A., Stevenson D. K. Sources of carbon monoxide (CO) in biological systems and applications of CO detection technologies. Semin Perinatol. 1994 Feb;18(1):2–10. [PubMed] [Google Scholar]
- Rotenberg M. O., Maines M. D. Isolation, characterization, and expression in Escherichia coli of a cDNA encoding rat heme oxygenase-2. J Biol Chem. 1990 May 5;265(13):7501–7506. [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Sun Y., Rotenberg M. O., Maines M. D. Developmental expression of heme oxygenase isozymes in rat brain. Two HO-2 mRNAs are detected. J Biol Chem. 1990 May 15;265(14):8212–8217. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Biro P., Cohen T., Müller R. M., Shibahara S. Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem. 1988 Feb 1;171(3):457–461. doi: 10.1111/j.1432-1033.1988.tb13811.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga T., Sassa S., Kappas A. Purification and properties of bovine spleen heme oxygenase. Amino acid composition and sites of action of inhibitors of heme oxidation. J Biol Chem. 1982 Jul 10;257(13):7778–7785. [PubMed] [Google Scholar]