Figure 2. Binding of citrulline versus arginine peptide side chains in the P4 pocket of HLA-DR proteins.
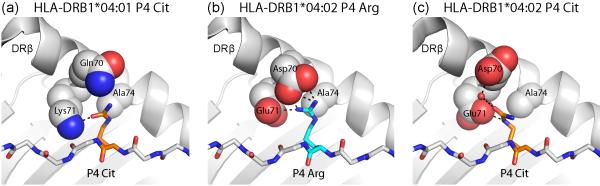
Arginine can be modified enzymatically to citrulline, resulting in loss of its positive charge. (a) The P4 pocket of the RA-associated DRB1*04:01 molecule can accommodate citrulline, which forms a hydrogen bond to DRβ Lys71. In contrast, arginine cannot be accommodated, due to the positive charge of DRβ Lys71 (PDB ID 4MCY). (b, c) The DRB1*04:02 protein is not associated with susceptibility to RA and differs at positions 70 and 71 from RA-associated HLA-DR molecules (PDB ID 4MDJ and 4MDI). Both arginine (b) and citrulline (c) can be accommodated in the P4 pocket. Arginine forms salt bridges with DRβ Glu71 and Asp70 (b), and citrulline forms hydrogen bonds with these two DR residues (c).
