Abstract
Background
Soluble vascular cell adhesion molecule-1 (sVCAM-1) is a marker of endothelial injury and a potent predictor of cardiovascular mortality in patients with kidney failure on dialysis. The longitudinal effects of dialysis on endothelial dysfunction and in particular the effects of renal transplantation on markers of endothelial function including sVCAM-1 have not been well characterized.
Methods
We used the Transplant Manitoba registry and biobank to assemble a retrospective cohort of all patients receiving a first kidney transplant between January 1, 2000, and December 31, 2005 (n=186). One hundred seventy-four patients had at least two serum samples pretransplant and at least two samples posttransplant. In total, 1,004 serial samples (median 5/patient) were analyzed. Factors associated with sVCAM-1 were examined using mixed linear models.
Results
The sVCAM-1 levels increased progressively on dialysis (0.15 [0.10 to 0.20] ng/mL/day; P<0.0001), fell significantly within 1 month after transplantation (−625 ng/mL/day; P<0.0001) and continued to fall thereafter (−0.23 [−0.34 to −0.12] ng/mL/day). Smoking and heart failure were associated with higher sVCAM-1 levels, whereas transplantation was associated with lower sVCAM-1 levels. The relationship between sVCAM-1 and transplantation was not changed by multivariate adjustment.
Conclusion
Endothelial injury worsens over time on dialysis but improves significantly after renal transplantation.
Keywords: Translational study, Vascular cell adhesion molecule, Cardiovascular disease
Supplemental digital content is available in the article.
Rates of cardiovascular events are 10 to 100 times higher in patients with end-stage renal disease (ESRD) than in the general population (1–3). Although traditional cardiovascular risk factors are prevalent in ESRD, they do not completely account for the elevated risk (4–7), suggesting that other mechanisms may be important. These mechanisms may include volume overload, diastolic dysfunction, vascular calcification, inflammation, oxidative stress, and endothelial dysfunction (8, 9). Of these, endothelial dysfunction represents an intriguing mechanism for explaining the excess cardiovascular risk in patients with ESRD because the endothelium is believed to play a central role in vascular homeostasis (9, 10).
Expression of adhesion molecules on the surface of activated endothelial cells is believed to be a critical step in the development of atherosclerosis, mediating recruitment of inflammatory cells into the vascular wall (11). In experimental atherosclerosis, for example, endothelial cells express vascular cell adhesion molecule-1 (VCAM-1) before monocytes or macrophages appear in the subendothelium. After activation, cell adhesion molecules are shed in soluble form from endothelial cells and can be measured in plasma. Soluble VCAM-1 (sVCAM-1) levels seem to correlate well with membrane bound VCAM-1 and VCAM messenger RNA expression; sVCAM-1 is thus considered a marker of endothelial activation (12, 13). Several cross-sectional analyses have shown that levels of sVCAM-1 are much higher in ESRD patients in comparison to the general population (14–19). These studies, however, do not provide information about the longitudinal evolution of endothelial injury in patients on dialysis and after transplantation.
The objective of this study was to test whether endothelial injury, as measured by serial sVCAM-1 measurements, worsens with time on dialysis and improves after transplantation. Furthermore, we set out to identify patient and dialysis characteristics associated with changes in endothelial injury over time.
RESULTS
All patients who received a first kidney transplant in Manitoba between January 1, 2000, and December 31, 2005 (n=186) were eligible for inclusion. Of the 186 transplanted patients, 12 were excluded: 10 patients were preemptive transplant recipients without exposure to dialysis and two declined consent. One hundred and seventy-four recipients (174) had at least two samples obtained in both dialysis and posttransplant periods; thus each patient had a minimum of four total measures of sVCAM-1 (Fig. 1).
FIGURE 1.
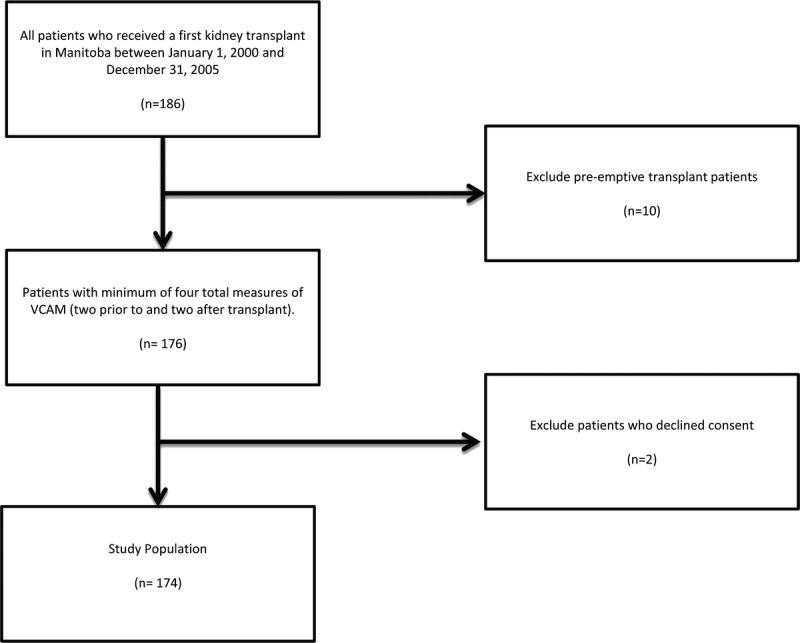
Derivation of study cohort.
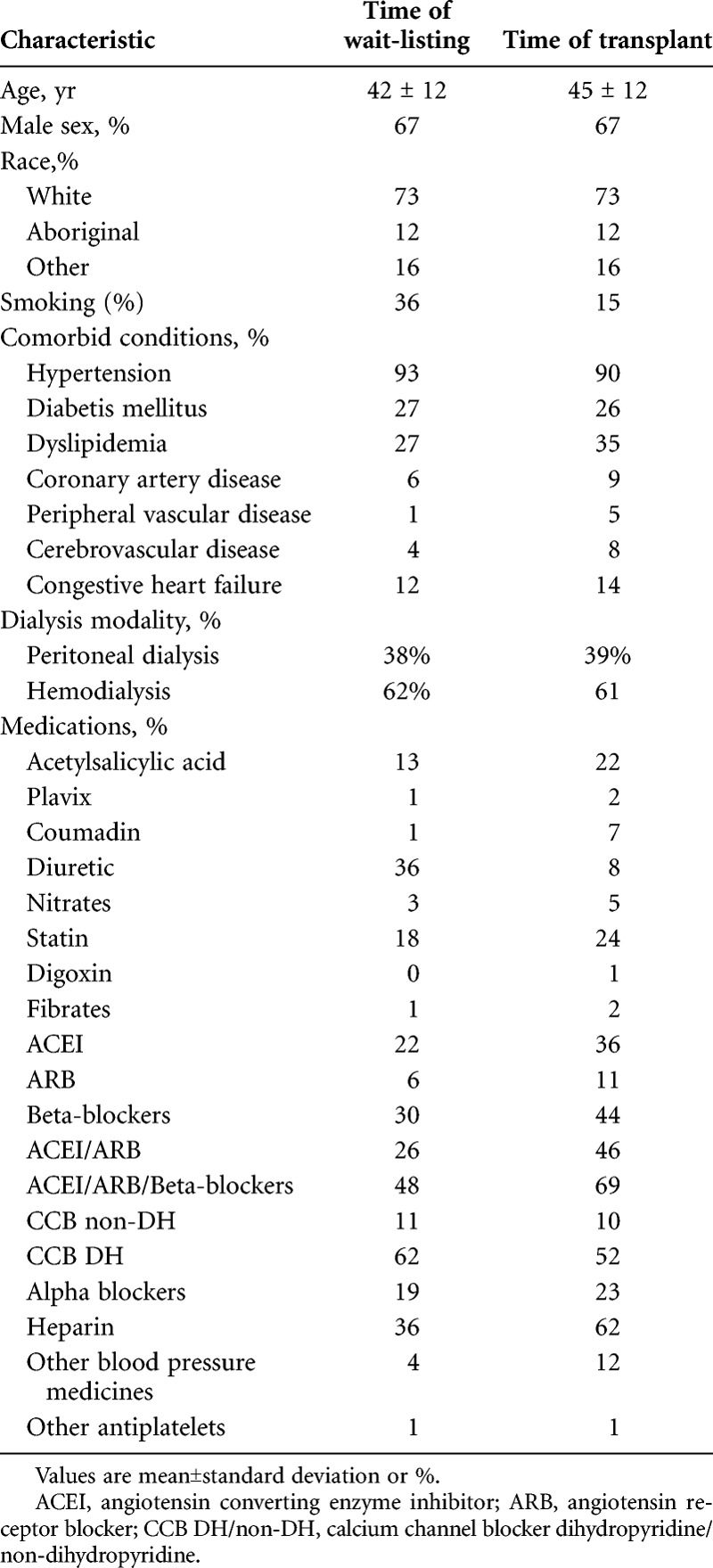
Characteristics of the study population at time of transplant wait listing and at time of transplant are presented in Table 1. Most patients were male (67%) and white. The median wait time for kidney transplantation was approximately 3 years from the time of transplant wait listing. Glomerulonephritis and diabetic nephropathy were the most common causes of ESRD. Twenty-seven percent were diabetic patients and 12% had congestive heart failure (CHF) at the time of transplant wait listing. The percentage of smokers decreased from 36% to 15% from time of dialysis wait listing to transplantation.
TABLE 1.
Patient characteristics at time of transplant wait-listing and at transplant
Evolution of sVCAM-1 Over Time and Impact of Transplantation
To correctly account for the unequal number of sVCAM-1 observations in each patient (see methods”” for explanation), the changes in sVCAM-1 over time and the impact of transplantation were analyzed using a mixed linear model (Table 2, Fig. 2). The model fitted is sVCAM-1=b0+b1×Time+b2×Transplant+b3×(Transplant×time), where b0 is the intercept (i.e., expected VCAM value at Time=0, just before transplantation), Time is the number of days before or after transplantation, “”Transplant is an indicator variable for transplantation status (0=pretransplant; 1=posttransplant), and the interaction term (Transplant×Time) describes whether the rate of change in VCAM is different in the pretransplant versus posttransplant periods. Importantly, the interaction coefficient b3 is highly statistically significant, indicating that the pretransplant and posttransplant rates of change of VCAM are indeed truly different.
TABLE 2.
Mixed-linear model describing the impact of time and transplantation on sVCAM-1 levels Variablea
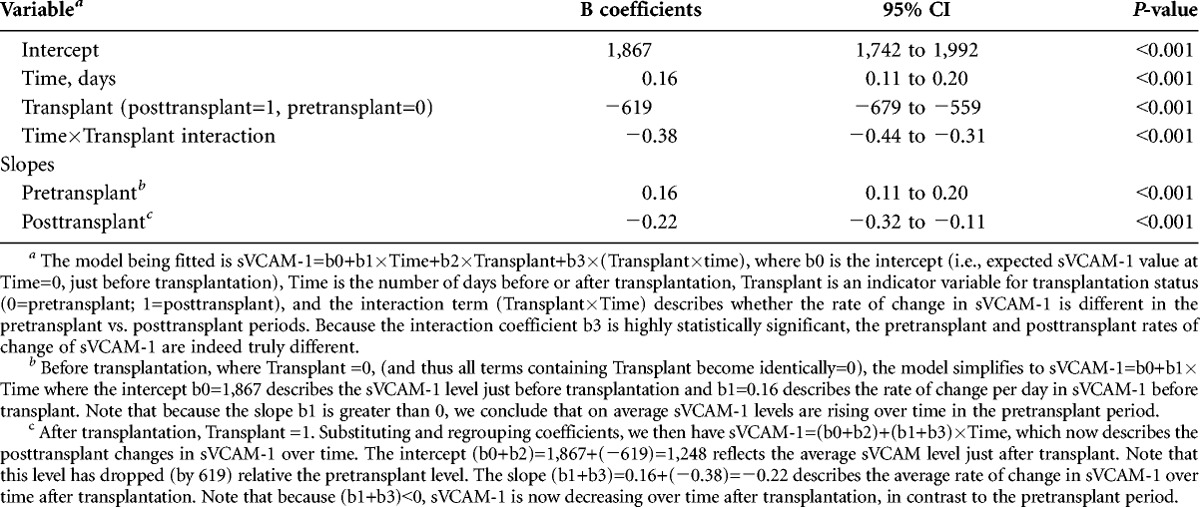
FIGURE 2.
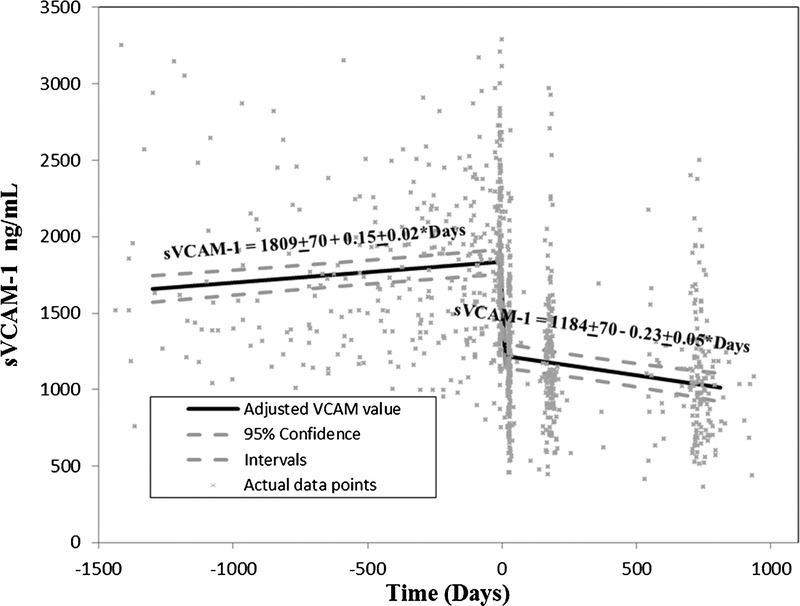
VCAM-1 levels over time on dialysis and after kidney transplantation. Time of transplant is designated as time zero. VCAM-1, vascular cell adhesion molecule-1.
Before transplantation (where “”Transplant =0, and all terms containing “”Transplant become identically=0), the model simplifies to sVCAM-1= b0 + b1×Time, where the intercept b0=1,867 ng/mL describes the sVCAM-1 level just before transplantation and b1=0.16 ng/mL per day describes the rate of change per day in sVCAM-1 before transplant. Because the slope b1 is greater than 0, we conclude that the average sVCAM-1 levels rise over time in the pretransplant period.
After transplantation, “”Transplant=1. Substituting and regrouping, the model becomes sVCAM-1= (b0+b2)+(b1+b3)×Time, which now describes the posttransplant changes in sVCAM-1 over time. The intercept (b0+b2)=1,867+(−619)=1,248 ng/mL reflects the average sVCAM-1 level just after transplant. Note that this level has dropped (by 619 ng/mL) relative the pretransplant level. The slope (b1+b3)=0.16+(−0.38)=−0.22 ng/mL per day describes the average rate of change in sVCAM-1 over time after transplantation. Note that because (b1+b3)<0, sVCAM-1 decreases over time after transplantation, in contrast to the pretransplant period.
Adjustment for Additional Factors Associated With Changes in sVCAM-1 Levels
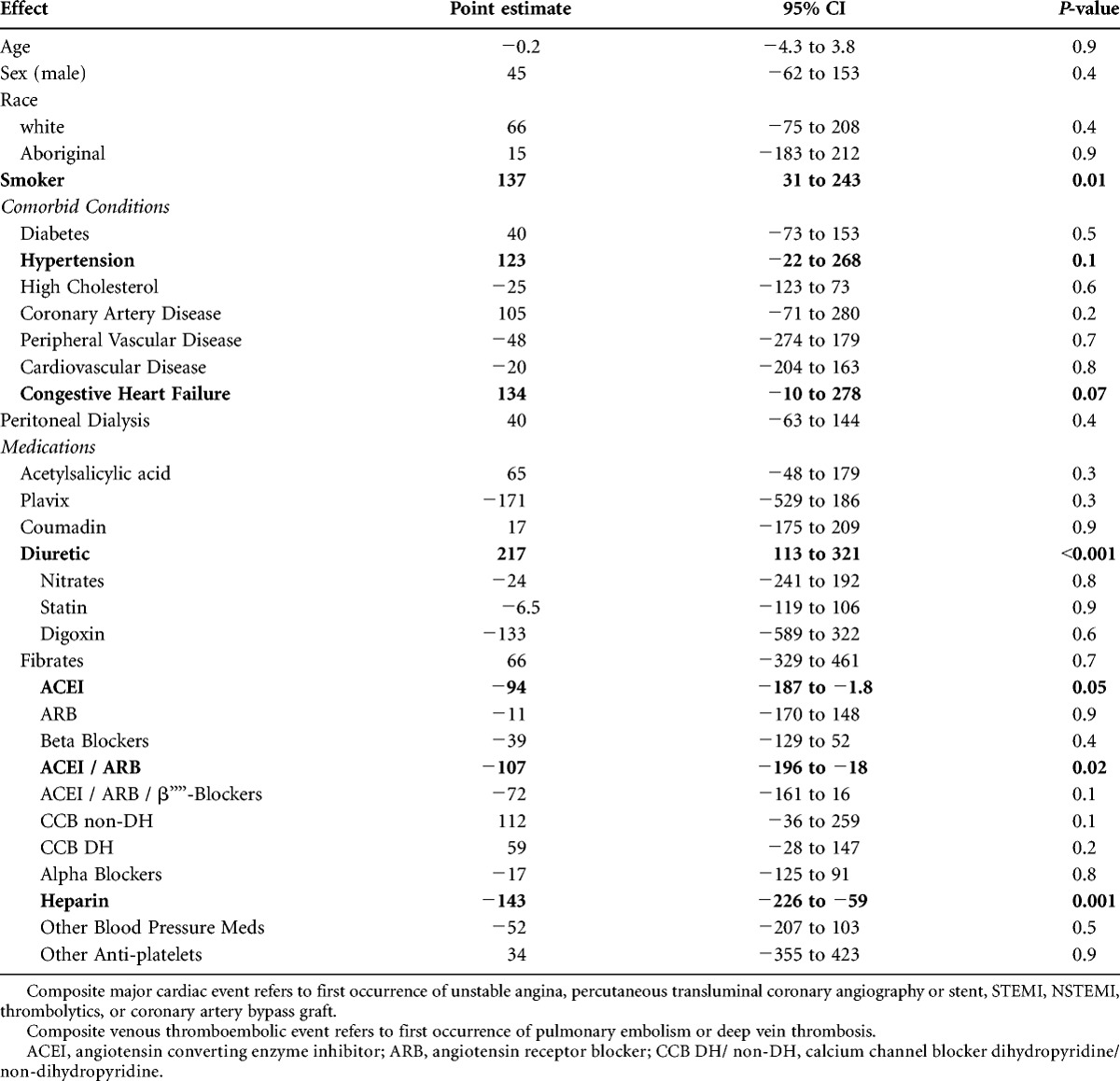
Additional univariable factors associated with changes in sVCAM-1 levels were determined using a mixed linear model and are presented in Table 3. Each of the univariable coefficients in Table 3 reflects the difference in mean sVCAM-1 level between the states of a categorical value. For example, the positive coefficient for Smoker in Table 2 indicates that mean sVCAM-1 levels were 137 ng/mL higher in smokers than in nonsmokers. Smoking status, hypertension, pretransplant heart failure, and diuretic use (a probable surrogate for hypertension and heart failure) were all associated with higher sVCAM-1 levels (positive coefficients) at the P less than 0.1 level. Conversely, angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) use and prior heparin exposure were all associated with a lower sVCAM-1 level (negative coefficients).
TABLE 3.
Factors associated with change in sVCAM-1 levels in patients undergoing kidney transplant on univariate analysis
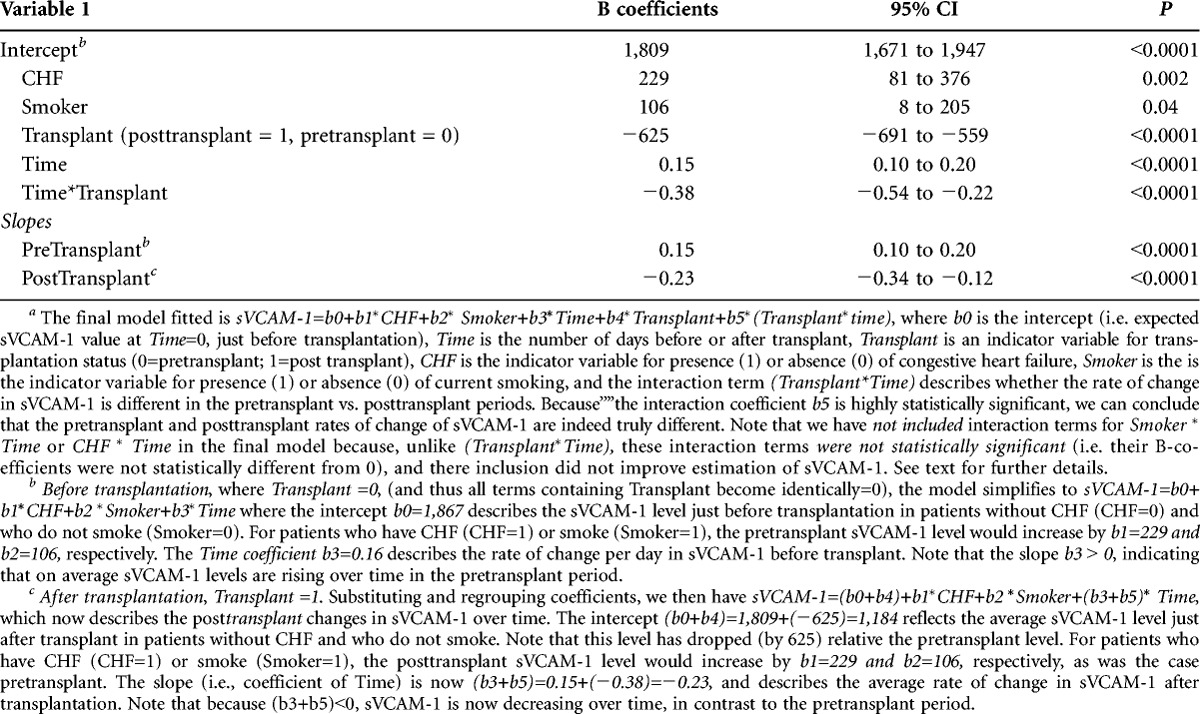
We then tested the hypothesis that the impact of these additional factors might alter the relationship between sVCAM-1 and transplantation by (1) adding as covariates all the variables identified as significant at the P less than 0.1 level in Table 3 to the mixed linear model described in Table 2; and (2) examining all first order interactions between each of these additional variables and time (slope effects), as well as each of these variables and Transplant status (intercept effects) (see “”Methods). We then simplified the model by eliminating terms with nonsignificant coefficients. Only pretransplant heart failure, smoking status, Transplant status, Time and Time×Transplant were retained in the final model shown in Table 4, which thus takes the form sVCAM-1=b0+b1×CHF+b2×Smoker+b3×Time+b4×Transplant+b5×(Transplant×time). The variables b0, Time, Transplant and (Transplant×Time) are as previously defined, CHF is an indicator variable for presence (1) or absence (0) of congestive heart failure, and Smoker is the is the indicator variable for presence (1) or absence (0) of current smoking. As expected, heart failure and smoking were associated with higher average sVCAM-1 levels (+229 and +106 ng/mL, respectively). Because the interaction terms Smoker×Transplant and CHF×Transplant were not statistically significant (and thus excluded from Table 4), we conclude that the increases associated with CHF and smoking were similar before and after transplantation. Furthermore, because the Smoker×Time and CHF×Time interaction terms were not significant, neither smoking nor CHF status seemed to influence the slope of sVCAM-1 over time. Of note, the coefficients for Transplant, Time, and Time×Transplant were nearly identical to those of the unadjusted model (Table 2), further suggesting that the impact of transplant on sVCAM-1 changes was independent of the other variables.
TABLE 4.
Multivariate mixed linear model analysisa (adjusting for all covariates and rate interactions with P value <0.1)
The changes in sVCAM-1 over time described in the tables can be summarized graphically (Fig. 2; Figure S1, SDC, http://links.lww.com/TP/A980). As noted, sVCAM-1 rose progressively during the pretransplant period at an average rate of +0.15 ng/mL per day. One month after transplantation, sVCAM-1 dropped on average 625 ng/mL and then continued to decline at a rate of −0.23 ng/mL per day subsequently. It is important to note that these changes before and after transplantation are occurring in each individual (i.e., these reflect average within individual changes) (Figure S1, SDC, http://links.lww.com/TP/A980).
DISCUSSION
In this longitudinal study of patients on dialysis waitlisted for kidney transplantation, we found that sVCAM-1 levels rose progressively with time on dialysis but then dropped significantly 1-month post–kidney transplantation and continued to decline out to 24 months. These findings are novel and illustrate progression of endothelial injury in patients with ESRD and amelioration after renal transplantation.
Our longitudinal observations strengthen the evidence from earlier cross-sectional studies (14, 16, 18, 19). In aggregate, these earlier studies suggested that sVCAM-1 level increased as kidney failure worsened and decreased after transplantation. For example, Vaccaro et al. (18) compared sVCAM-1-1 levels in 32 patients with predialysis chronic kidney disease (CKD), 30 on maintenance hemodialysis, 36 after kidney transplantation, and 28 “normal” controls, and found that sVCAM-1 increased progressively from controls to kidney transplant, to predialysis CKD, with the highest sVCAM-1-1 in hemodialysis patients. However, because these studies were cross-sectional and did not measure changes in sVCAM-1 in the same patient at different time points, temporal changes in sVCAM-1 are only inferred. For example, in the study by Vaccaro et al., the results could potentially have been confounded by differences other than disease stage or transplant status between the patient groups sampled. Our study is unique in having repeated measures of sVCAM-1 in the same patients over time and over the transition to transplantation. Thus, it does not suffer the design limitation of cross-sectional studies and provides direct verification that sVCAM-1 increases over time on dialysis and the drops after kidney transplantation.
The changes in sVCAM-1 in our study are consistent with the known epidemiology of cardiovascular disease in ESRD. It has been shown that cumulative exposure to dialysis is an independent risk factor for cardiovascular events both during dialysis and after renal transplantation (5, 6, 20–24). Conversely, restoration of renal function with renal transplantation decreases CVD event rate (3, 5, 25). Indeed, preemptive transplantation, in which dialysis exposure is avoided altogether, is associated with superior cardiovascular outcomes (24, 26–28). Importantly, others have observed an association between sVCAM-1 levels and clinical events (29, 30). This observation strengthens the inference of a potential causal link between sVCAM-1 and outcomes in CKD. Taken together, the congruence between the sVCAM-1 changes reported in our study and well-established epidemiological trends in clinical outcomes observed by others suggests that endothelial injury may be a major driver in the evolution of cardiovascular disease in ESRD.
In our analysis of factors associated with sVCAM-1 levels, we found that smoking status, diuretic use, and CHF were significantly associated with higher sVCAM-1 levels, findings consistent with cross-sectional studies in non-ESRD cohorts (31, 32). However, other comorbid conditions, including diabetes, hypertension, and dyslipidemia were not associated with sVCAM-1, again consistent with cross-sectional data in other renal populations (14, 16) but in contrast to observations in nonrenal cohorts (31, 32). Somewhat unexpectedly, medication use was not strongly associated with sVCAM-1; although use of ACEIs or ARB demonstrated a univariate association with lower sVCAM-1 level, this association disappeared after adjustment for other factors. These findings are consistent with other cross-sectional analyses however (8, 33). Importantly, because we modeled hypertension, diabetes, dyslipidemia, and medications in a time-dependent fashion, we conclude that changes in sVCAM-1 were not attributable to time-dependent changes in these variables.
We cannot easily identify the proximate causes of the longitudinal changes in sVCAM-1 in the present study. In theory, the observed changes could result from altered production or altered elimination. Although the mechanisms of elimination of adhesion molecules are not well understood, it is unlikely that alterations in renal filtration (i.e., gradual loss of residual renal function over time during dialysis followed by restoration of GFR after transplant) are responsible because sVCAM-1 is too “”a large molecule (110 kDa) to be readily filtered by the kidney. More likely, the changes reflect worsening endothelial function over time on dialysis followed by amelioration after transplantation. It is known that loss of functioning renal mass results in retention of nitrogenous compounds normally eliminated by the kidney. In vitro studies have demonstrated that exposure of cultured endothelial cells to uremic serum results in increased expression of VCAM (33, 34). Furthermore, investigators found that there was a significant increase in sVCAM-1 in both serum and aortic smooth muscle after induction of uremia in mice. Although most retained uremic compounds remain uncharacterized, some, such as asymmetric dimethyl arginine and homocysteine, are known to impair or injure endothelium (35–37). In addition, retained nitrogenous compounds, such as urea, have been shown to promote carbamylation of low-density lipoprotein, which may promote atherosclerosis and stimulate macrophage cytokine production and myeloperoxidase activity, contributing to both inflammation and oxidative stress (38, 39). Higher molecular weight toxins may also play a role. Studies have suggested that in renal failure, retention of oxidatively damaged proteins leads to scavenger receptor mediated activation of macrophages and other antigen-presenting cells (40). Chronic macrophage activation may then lead by means of multiple pathways to systemic inflammation and oxidative stress, both of which may contribute directly to endothelial activation. After a successful kidney transplantation, clearance of retained toxins would be expected to reverse the proinflammatory and prooxidative milieu, potentially leading to improvement in endothelial function. Although this schema is conceptually attractive, large gaps remain in our understanding of the specific mechanisms involved.
Our observations, in the context of the conceptual framework proposed above, have implications for future research. Study of the temporal relationship between uremic toxins, inflammation, oxidative stress, and endothelial markers during dialysis and after transplantation may help elucidate the primary causes of endothelial dysfunction in ESRD. Moreover, studies are needed to evaluate the relationship between sVCAM-1 and physiologic measures of endothelial function, such as brachial artery reactivity or peripheral arterial tonometry. Validation of sVCAM-1 and noninvasive testing of vascular reactivity as prognostic markers for clinical events may in the future allow for early detection of endothelial activation and cardiovascular disease in patients with kidney disease.
The main strengths of our study were a well-characterized cohort of wait-listed patients, and a longitudinal, rather than cross-sectional, design with repeated measures of sVCAM-1 before and after transplantation, permitting analysis of within patient changes in sVCAM-1. The results of our study support existing literature on the role of sVCAM-1 as an indicator of endothelial activation and provide novel insight into the temporal evolution of sVCAM-1 as patients transition from dialysis to transplantation.
Our study also has limitations. We studied only “”waitlisted patients who ultimately received a transplant, and caution should be taken in applying our results to non–wait-listed patients. We could not obtain physiological measures of endothelial function (e.g., brachial artery dilatation) to complement our sVCAM-1 measurements. Finally, as our study was not powered for analysis of clinical events (e.g., cardiovascular events, death), we could not explore an association between sVCAM-1 and posttransplant outcomes in the study cohort.
In conclusion, endothelial injury increases over time on dialysis but improves after restoration of renal function by renal transplantation. These changes provide an attractive biological explanation for the accelerated cardiovascular disease experienced by dialysis patients as well as the improved cardiovascular risk observed after transplantation. Future research should focus on discovering which uremic toxins are primarily responsible for altered endothelial function, as this could uncover new targets for therapy, identify novel prognostic markers, and contribute to a greater biological understanding of vascular disease in ESRD.
MATERIALS AND METHODS
The study protocol was approved by the institutional review boards of the University of Manitoba and the Health Sciences Centre, Winnipeg, Manitoba, Canada.
Study Design
In this observational cohort study, we studied prospectively collected frozen serum samples of dialysis patients wait listed for kidney transplantation in Manitoba, Canada. Biobanking of frozen serum samples has been performed systematically per protocol on all wait listed dialysis and renal transplant patients in Manitoba since 1999. All patients were approached by the study nurse to provide informed consent for use of the samples for the study, with the exception of patients who had died; in these cases, a waiver of consent was granted by the institutional review boards.
Patient Selection
Figure 1 outlines the derivation of the study cohort. All patients who received a first kidney transplant in Manitoba between January 1, 2000, and December 31, 2005 (n=186) were eligible for inclusion. Of the one hundred and eighty six (186) transplanted patients, 10 patients were preemptive transplant recipients and two declined consent. One hundred and seventy-four recipients (174) had at least two samples obtained in both pretransplant and posttransplant periods; thus each patient had a minimum of four total measures of sVCAM-1.
sVCAM-1 Measurement
All serum samples were drawn immediately predialysis in hemodialysis patients or at an outpatient clinic visit in peritoneal dialysis and transplanted patients. Samples were centrifuged and aliquoted immediately into cryovials for storage at −70°C. Biomarkers were measured from thawed sera using commercially available enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN). All measurements were in duplicate.
Clinical Data
A research nurse reviewed all inpatient and outpatient records and abstracted the data elements of interest. Comorbidities were adjudicated by one of us (J.B.) according to a priori definitions. All reviewers were blinded to sVCAM-1 levels.
Baseline variables abstracted at time of wait-listing included demographic data (age, sex, ethnicity, renal disease), known comorbid conditions (history of cardiovascular disease, history of other major organ system disease, diabetes), known cardiovascular risk factors (e.g., hypertension, cholesterol level, smoking status), and additional potential confounders (e.g., medications antiplatelet agents, anti-hypertensives, ACEI or ARB, and immunosuppressive medications). Follow-up values within ô±3 months of the date of each serum sample were abstracted for all variables which could change over time (e.g., hypertension, medications). Medication doses were not recorded, only whether the patient was on the medication or not was abstracted.
Statistical Analysis
Continuous variables are summarized as mean (standard deviation) or median (interquartile range) depending on distribution; categorical variables are expressed as percentages.
Mixed linear modeling was used to examine changes in sVCAM-1 as a function of time and transplantation. Mixed linear modeling was chosen over general linear modeling or repeated measures analysis of variance because it does not require observations to be independent or have constant variance, it does not require all subjects to have the same number of observations, and it is fairly robust to departures from normality in the dependent variable (sVCAM-1) (41). Time was modeled explicitly as a random factor, with the origin of the time scale (i.e., time =0) set as the date of transplantation. This choice of origin has no influence on the relationships between the independent variables and cVCAM-1; however, it does mean that measures or events occurring before transplantation showed negative times on this scale, and all measures or events occurring after transplantation showed positive times. Transplantation was modeled as a state transition variable taking the value=0 for all time points before transplantation and value=1 for all time points after transplantation. Other time-varying exposures including hypertensive status, dialysis modality, and medications, were modeled as time-dependent variables. Age, gender, and baseline comorbid conditions were treated as fixed factors.
The base model fitted took the form sVCAM-1= b0+b1×Time+b2×Transplant+b3×(Transplant×time), where b0 is the intercept (i.e., expected sVCAM-1 value at Time=0, just before transplantation), Time is the number of days before or after transplant, and Transplant is an indicator variable for transplantation status (0=pretransplant; 1=posttransplant). The interaction term (Transplant×Time) explicitly models whether the rate of change in sVCAM-1 is different in the pretransplant versus posttransplant periods; a statistically significant coefficient indicates that the slopes (i.e., rates of change) are significantly different in each period.
To address whether other patient characteristics influenced the association between sVCAM-1, Transplant, and Time, we constructed enriched models in a forward stepwise fashion. First, we determined which additional factors influenced sVCAM-1 level on univariable testing. We then added these variables to the base model to see if they remained statistically significant at the P less than 0.1 level. The initial enriched model explored was
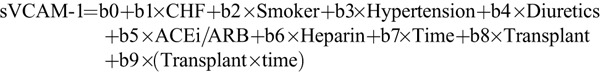
Because only CHF, Smoker, and the base model variables were statistically significant at the P less than 0.1 level, only these terms were retained in the model. In the next step, to explore the question of whether the effect of the additional variables CHF and Smoking were different pretransplant versus posttransplant, we examined the first-order interaction terms CHF×Transplant and Smoker×Transplant. Because neither interaction was statistically significant, these terms were dropped from the model. In the last step, to address the question of whether CHF or smoking influenced the rate of change of sVCAM-1, we examined the interaction terms CHF×Time and Smoker×Time. Again, as neither interaction was statistically significant, the terms were dropped from the model. The final adjusted model therefore took the form:
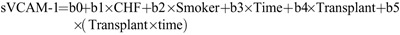
We additionally conducted two sensitivity analyses. In the first analysis, we reintroduced variables and interaction terms excluded at an earlier stage in the model building process to see if they would now alter the model specification. In the second analysis, we used a stepwise backward model building approach where the starting point was a fully specified model including all potentially significant variables and all first-order interactions with Time and Transplant. Individual terms were then sequentially removed until no further terms could be removed without significantly altering model fit. In both approaches, the final model was identical, and thus only the main analysis is presented in the article. All analyses were performed using SAS v9.2.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Melanie Zarrillo RN for her chart reviews.
Footnotes
This study was funded by a grant from Manitoba Health Research Council (J.B. and C.R.).
The authors declare no conflicts of interest.
J.B. and C.R. conceived and designed the study, conducted the research, and contributed to the analysis of study results. N.T. contributed to the analysis of data and article writing. K.M. contributed to the analysis of data and revision of the article. Y.X. contributed to the revision and preparation of the article. B.H. was responsible for construction of the statistical models and contributed to the preparation of the article. P.N. oversaw measurement of sVCAM-1 and data collection. D.R. contributed to the design and conception of study and article preparation. M.S. and P.K. contributed to interpretation of the data and article preparation.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1. Lindner A, Charra B, Sherrard DJ, et al. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med 1974; 290: 697. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Organ Replacement Register. Volume 1: Dialysis and renal transplantation Ottawa, Canada: Canadian institute of health information, 2001. [Google Scholar]
- 3. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32 (5 Suppl 3): S112. [DOI] [PubMed] [Google Scholar]
- 4. Parfrey PS, Foley RN, Harnett JD, et al. Outcome and risk factors of ischemic heart disease in chronic uremia. Kidney Int 1996; 49: 1428. [DOI] [PubMed] [Google Scholar]
- 5. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the american heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 2003; 108: 2154. [DOI] [PubMed] [Google Scholar]
- 6. Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol 2000; 11: 1735. [DOI] [PubMed] [Google Scholar]
- 7. Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005; 353: 238. [DOI] [PubMed] [Google Scholar]
- 8. Stenvinkel P, Carrero JJ, Axelsson J, et al. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 2008; 3: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation 2007; 116: 85. [DOI] [PubMed] [Google Scholar]
- 10. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004; 109 (23 Suppl 1): III27. [DOI] [PubMed] [Google Scholar]
- 11. Hansson G. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol 2001; 21: 1876. [DOI] [PubMed] [Google Scholar]
- 12. Pigott R, Dillon LP, Hemingway IH, et al. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun 1992; 187: 584. [DOI] [PubMed] [Google Scholar]
- 13. De Caterina R, Basta G, Lazzerini G, et al. Soluble vascular cell adhesion molecule-1 as a biohumoral correlate of atherosclerosis. Arterioscler Thromb Vasc Biol 1997; 17: 2646. [DOI] [PubMed] [Google Scholar]
- 14. Liakopoulos V, Eleftheriadis T, Kyropoulos T, et al. Hemodialysis procedure does not affect the levels of sICAM-1 and sVCAM-1 in patients with end stage renal disease. Ren Fail 2005; 27: 315. [PubMed] [Google Scholar]
- 15. Bonomini M, Reale M, Santarelli P, et al. Serum levels of soluble adhesion molecules in chronic renal failure and dialysis patients. Nephron 1998; 79: 399. [DOI] [PubMed] [Google Scholar]
- 16. Papayianni A, Alexopoulos E, Giamalis P, et al. Circulating levels of ICAM-1, VCAM-1, and MCP-1 are increased in haemodialysis patients: association with inflammation, dyslipidaemia, and vascular events. Nephrol Dial Transplant 2002; 17: 435. [DOI] [PubMed] [Google Scholar]
- 17. Papagianni A, Dovas S, Bantis C, et al. Carotid atherosclerosis and endothelial cell adhesion molecules as predictors of long-term outcome in chronic hemodialysis patients. Am J Nephrol 2008; 28: 265. [DOI] [PubMed] [Google Scholar]
- 18. Vaccaro F, Mule G, Cottone S, et al. Circulating levels of adhesion molecules in chronic kidney disease correlate with the stage of renal disease and with C-reactive protein. Arch Med Res 2007; 38: 534. [DOI] [PubMed] [Google Scholar]
- 19. Cottone S, Palermo A, Vaccaro F, et al. Inflammation and endothelial activation are linked to renal function in long-term kidney transplantation. Transpl Int 2007; 20: 82. [DOI] [PubMed] [Google Scholar]
- 20. Ducloux D, Kazory A, Chalopin JM. Predicting coronary heart disease in renal transplant recipients: a prospective study. Kidney Int. 2004; 66: 441. [DOI] [PubMed] [Google Scholar]
- 21. Iseki K, Tozawa M, Takishita S. Effect of the duration of dialysis on survival in a cohort of chronic haemodialysis patients. Nephrol Dial Transplant 2003; 18: 782. [DOI] [PubMed] [Google Scholar]
- 22. Cosio FG, Alamir A, Yim S, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int 1998; 53: 767. [DOI] [PubMed] [Google Scholar]
- 23. Rigatto C, Foley RN, Kent GM, et al. Long-term changes in left ventricular hypertrophy after renal transplantation. Transplantation 2000; 70: 570. [DOI] [PubMed] [Google Scholar]
- 24. Foley RN, Parfrey PS, Kent GM, et al. Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J Am Soc Nephrol 2000; 11: 912. [DOI] [PubMed] [Google Scholar]
- 25. Parfrey PS, Harnett JD, Foley RN, et al. Impact of renal transplantation on uremic cardiomyopathy. Transplantation 1995; 60: 908. [PubMed] [Google Scholar]
- 26. Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation 2002; 74: 1377. [DOI] [PubMed] [Google Scholar]
- 27. Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000; 58: 1311. [DOI] [PubMed] [Google Scholar]
- 28. Meier-Kriesche HU, Schold JD. The impact of pretransplant dialysis on outcomes in renal transplantation. Semin Dial 2005; 18: 499. [DOI] [PubMed] [Google Scholar]
- 29. Suliman ME, Qureshi AR, Heimburger O, et al. Soluble adhesion molecules in end-stage renal disease: a predictor of outcome. Nephrol Dial Transplant 2006; 21: 1603. [DOI] [PubMed] [Google Scholar]
- 30. Wang AY, Lam CW, Wang M, et al. Circulating soluble vascular cell adhesion molecule 1: relationships with residual renal function, cardiac hypertrophy, and outcome of peritoneal dialysis patients. Am J Kidney Dis 2005; 45: 715. [DOI] [PubMed] [Google Scholar]
- 31. Yin WH, Chen JW, Jen HL, et al. The prognostic value of circulating soluble cell adhesion molecules in patients with chronic congestive heart failure. Eur J Heart Fail. 2003; 5: 507. [DOI] [PubMed] [Google Scholar]
- 32. Andreassen AK, Nordoy I, Simonsen S, et al. Levels of circulating adhesion molecules in congestive heart failure and after heart transplantation. Am J Cardiol. 1998; 81: 604. [DOI] [PubMed] [Google Scholar]
- 33. Stinghen AE, Goncalves SM, Martines EG, et al. Increased plasma and endothelial cell expression of chemokines and adhesion molecules in chronic kidney disease. Nephron Clin Pract 2009; 111: c117. [DOI] [PubMed] [Google Scholar]
- 34. Serradell M, Diaz-Ricart M, Cases A, et al. Uremic medium causes expression, redistribution and shedding of adhesion molecules in cultured endothelial cells. Haematologica 2002; 87: 1053. [PubMed] [Google Scholar]
- 35. Zoccali C, Bode-Boger S, Mallamaci F, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 2001; 358: 2113. [DOI] [PubMed] [Google Scholar]
- 36. Mallamaci F, Zoccali C, Tripepi G, et al. Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney Int 2002; 61: 609. [DOI] [PubMed] [Google Scholar]
- 37. Bro S, Moeller F, Andersen CB, et al. Increased expression of adhesion molecules in uremic atherosclerosis in apolipoprotein-E-deficient mice. J Am Soc Nephrol 2004; 15: 1495. [DOI] [PubMed] [Google Scholar]
- 38. Wang Z, Nicholls SJ, Rodriguez ER, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med 2007; 13: 1176. [DOI] [PubMed] [Google Scholar]
- 39. Moeslinger T, Friedl R, Volf I, et al. Urea induces macrophage proliferation by inhibition of inducible nitric oxide synthesis. Kidney Int 1999; 56: 581. [DOI] [PubMed] [Google Scholar]
- 40. Galli F. Protein damage and inflammation in uraemia and dialysis patients. Nephrol Dial Transplant 2007; 22 (Suppl 5): v20. [DOI] [PubMed] [Google Scholar]
- 41. Norusis M. SPSS 17.0: Advanced stastical procedures companion. Upper Saddle River: Prentice Hall Press; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


