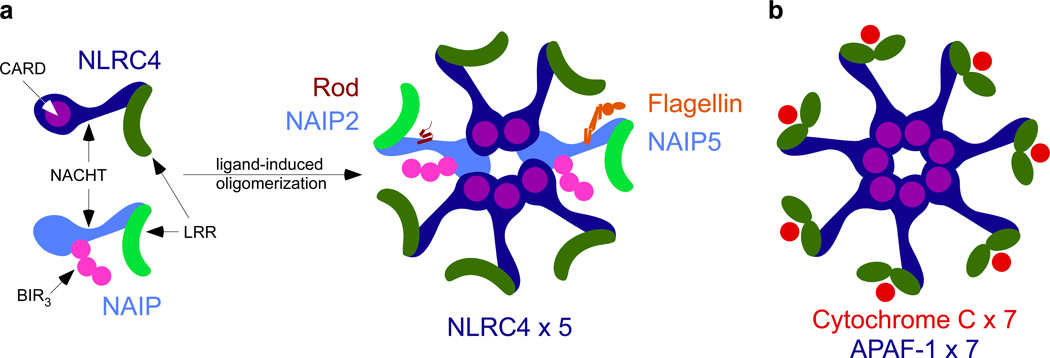
Figure 1. Structural and functional similarities between the APAF-1 apoptosome and the NAIP/NLRC4 inflammasome.
(A) Comparison of NAIP and NLRC4 NLR proteins. (B)The apoptosome is an oligomer of APAF-1, which consists of 3 distinct domains, an N-terminal caspase activation and recruitment domain (CARD, in purple), an expanded nucleotide binding domain (NBD, in blue), and WD40 repeats (green) at the C-terminus. The WD40 repeats bind cytochrome c (red) and oligomerize to form a homo-heptamer, with each APAF-1 bound to one cytochrome c. The NAIP/NLRC4 inflammasome can exist as a mixed oligomer. In a proposed heptamer formation, paralleling the apoptosome, different NAIPs (light blue) can bind their cognate ligand (rod-NAIP2, flagellin-NAIP5), recruiting NLRC4 (dark blue). This relieves autoinhbition by the LRR (green) and could twist the baculovirus inhibitor of apoptosis protein repeat (BIR, in pink) for potential interactions with NLRC4.