Figure 1. Clinical Trial Considerations Related to Comorbidities.
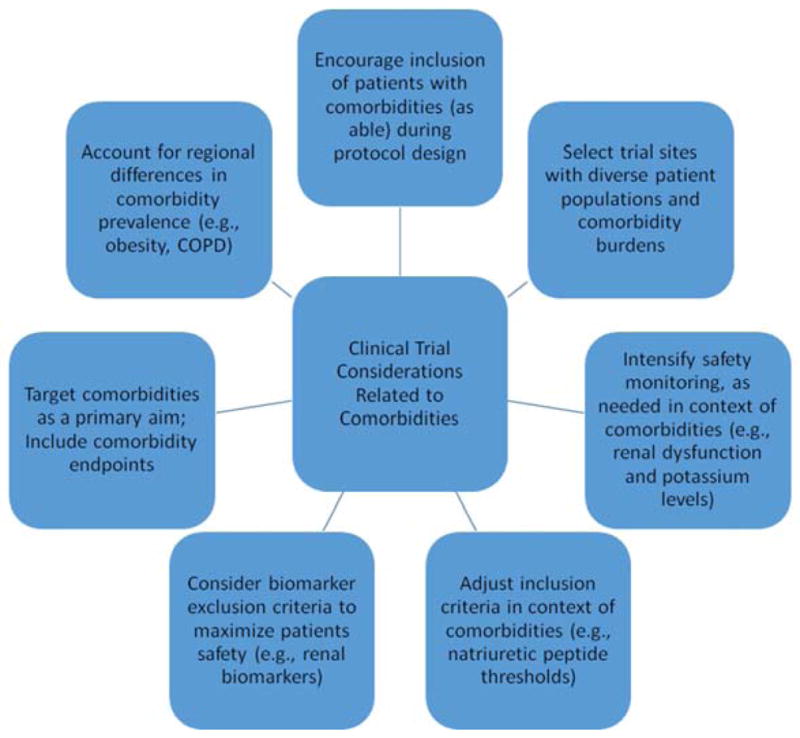
Trial protocols should consider feasibility of encouraging the inclusion of patients with comorbidities (as able). Trial sites should be selected on the basis, in part, of the comorbidity burden of the patient populations. Intensified safety monitoring may be needed in the context of comorbidities. Study entry criteria may need to be adjusted to acknowledge differences in biomarker thresholds in the context of comorbidities such as obesity and/or renal function. Exclusionary biomarker criteria (e.g., renal biomarkers) may offer mechanisms to support patient safety. As able, consider targeting comorbidities as a primary intervention and include comorbidity-specific trial endpoints (e.g., dyspnea relief and beta-blocker usage in context of COPD). Trialists should also take into account regional differences in the prevalence of comorbidities when performing sample size calculations and designing trials (e.g., differential event rates in the presence of comorbid diseases).
