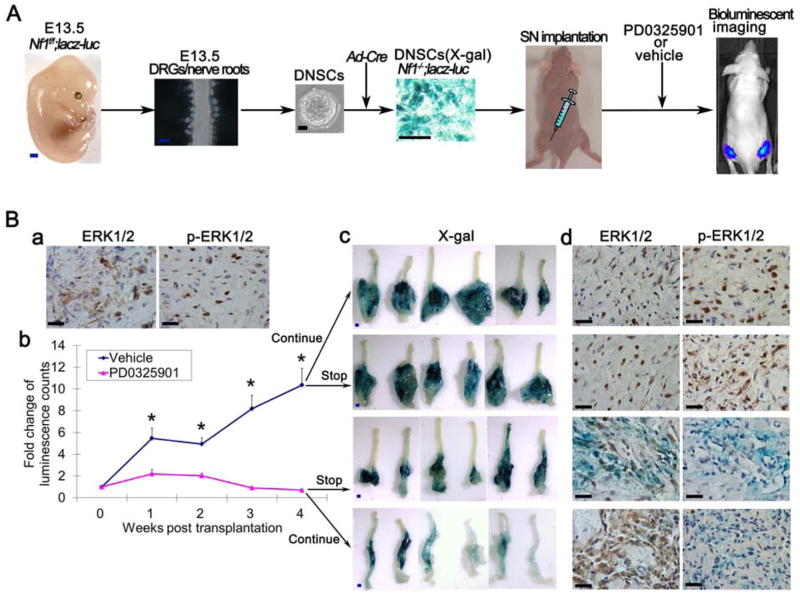
Figure 6. Therapeutic effect of MAPK signaling inhibitor on DNSC-derived plexiform neurofibroma.
(A) Diagram of experimental design for testing therapeutic effect of PD0325901 on plexiform neurofibroma.
(B) Immunostaining of ERK1/2 (total) and p-ERK1/2 (phosphorylated ERK) in E13.5 Nf1−/− DNSCs derived sciatic plexiform neurofibroma (a). Fold change of luminescence count measured during PD0325901 or vehicle treatment (b). After 4 weeks of treatment, both PD0325901 and vehicle treatment group were divided into two subgroups: discontinued PD0325901 or continued the PD0325901 and discontinued vehicle treatment or continued vehicle treatment subgroups for an additional 4 weeks. Tumors were then harvested for X-gal staining (c) and immunostaining for ERK1/2 and p-ERK1/2 (d). Statistics were represented as the mean ± SEM (* p < 0.05).
Scale bars: 500 μm for blue scale bars, 50 μm for the rest.
See also Figure S4.