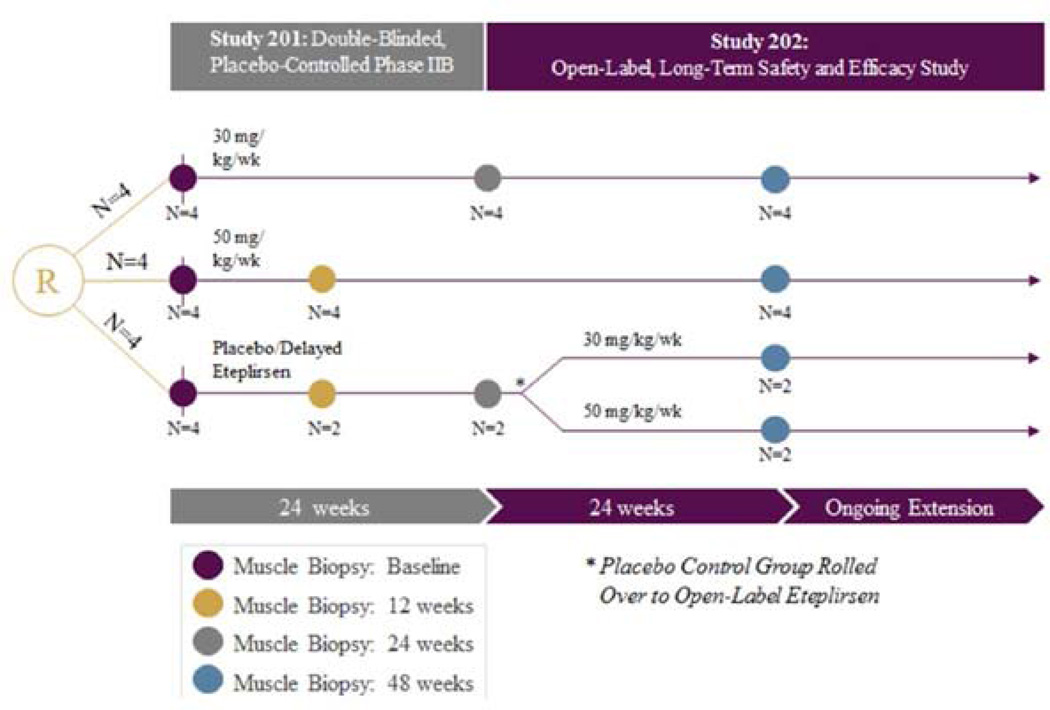
Figure 1.
Eteplirsen exon skipping study design. Twelve subjects with DMD were randomized (R) to 1 of 3 eteplirsen-treated cohorts in study 201: Cohort 1, received 30 mg/kg/wk; Cohort 2, 50 mg/kg/wk; and Cohort 3, placebo-treated. At week 25, Cohort 3, switched to open-label treatment, either 30 or 50 mg/kg/wk; thereafter referred to as “placebo-delayed”. Patients were maintained on the same starting dose of eteplirsen under the open label extension Study 202. Biceps biopsies were obtained on all patients at baseline and deltoid biopsies at week 48 for analysis of dystrophin expression. At week 12 biceps biopsies were obtained from patients in Cohort 2 (50 mg/kg/wk) and 2 Placebo-treated patients in Cohort 3. At week 24 biceps biopsies were obtained from patients in Cohort 1 (30 mg/kg/wk) and 2 placebo-treated patients in Cohort 3.