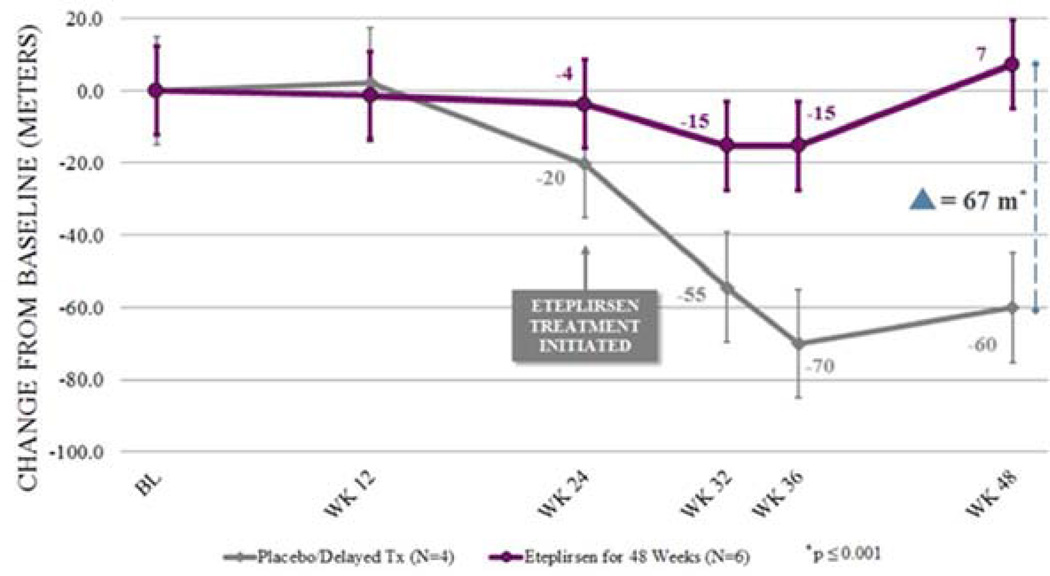
Figure 3.
Functional efficacy of eteplirsen. The purple line illustrates the change from baseline (BL) in distance walked on the 6-minute walk test (6MWT) over time for the 6 evaluable patients who received eteplirsen from the start of Study 201. The dark gray line represents change from baseline in distance walked on the 6MWT for the 4 patients who received placebo for the first 24 weeks and eteplirsen for the last 24 weeks. It is estimated from dystrophin expression studies and multiple biopsies that significant dystrophin was produced after 12 weeks. This is clearly indicated in the placebo/delayed cohort starting on eteplirsen at week 25 and stabilizing in function at week 36. Eteplirsen-treated cohorts beginning at study initiation maintained a plateau without decline through week 48, diverging from placebo/delayed patients at week 12. Two of the treated patients starting at week 1 lost ambulation before they produced significant dystrophin and are not represented on this graph.