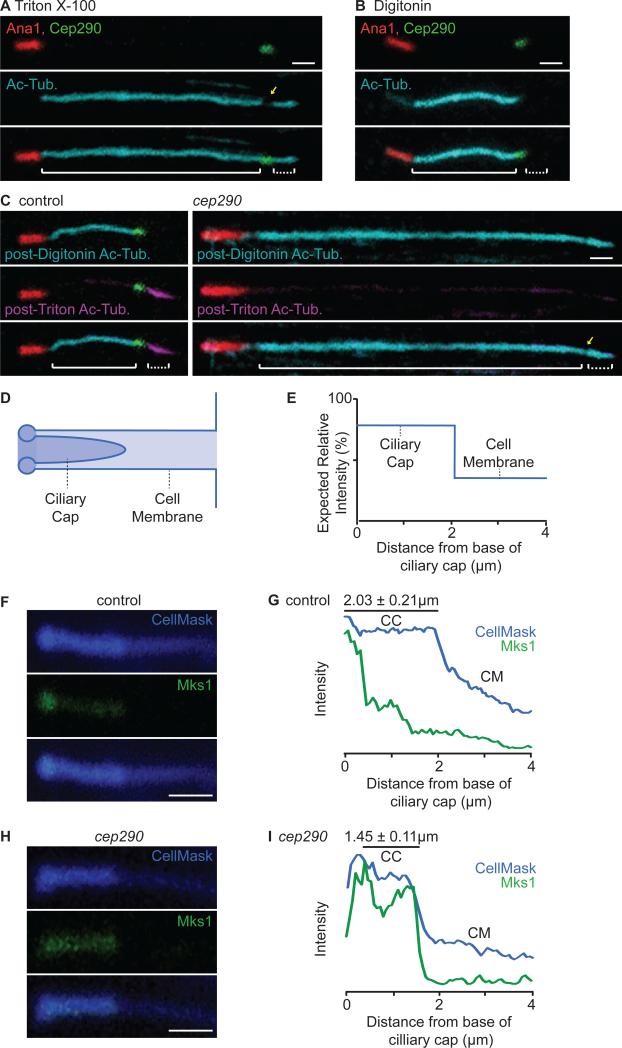
Figure 6. The Ciliary Cap is a Distinct Compartment that is Compromised in cep290mecH.
(A-B) Triton X-100 and Digitonin differentially permeabilize the cell and ciliary cap membranes. (A) Triton X-100 permeabilizes both the cell and ciliary cap membranes, allowing for anti-acetyl-tubulin staining of the entire axoneme. (B) Digitonin preferentially permeabilizes the cell membrane, allowing for anti-acetyl-tubulin staining of only the cytoplasmic axoneme. (C) Ciliary cap compartmentalization is compromised in cep290mecH. (C, left) In control spermatids, post-Digitonin staining specifically labels the cytoplasmic axoneme segment (cyan) while post-Triton X-100 staining specifically labels the ciliary cap axoneme (magenta) (N>70). (C, right) In cep290mecH, post-Digitonin staining labels both the cytoplasmic and ciliary cap axoneme segments (N≥60). (A, C) Yellow arrow, reduction in anti-acetyl-tubulin at the transition zone. Solid brackets, cytoplasmic axoneme; dotted brackets, ciliary cap axoneme. Note that all spermatids analyzed were at the same developmental stage; any difference in length displayed is within normal variation. (D) Illustration depicting the cell membrane invagination containing the ciliary cap and (E) expected CellMaskTM signal. (F-I) Ciliary cap length and Mks1-GFP distribution are abnormal in cep290mecH. (F-G) In control spermatids, CellMaskTM and Mks1-GFP mark the ciliary cap membrane and transition zone. (H-I) In cep290mecH, CellMaskTM marks a shorter ciliary cap and Mks1-GFP labeling is not focused at the base. (G, I) Mean signal was quantified along a 4 μm region distal to the ciliary cap base (N=10). CM, cell membrane invagination; CC, ciliary cap membrane. (A-C, F, H) Scale bar, 1 μm. Also, see Figure S3.