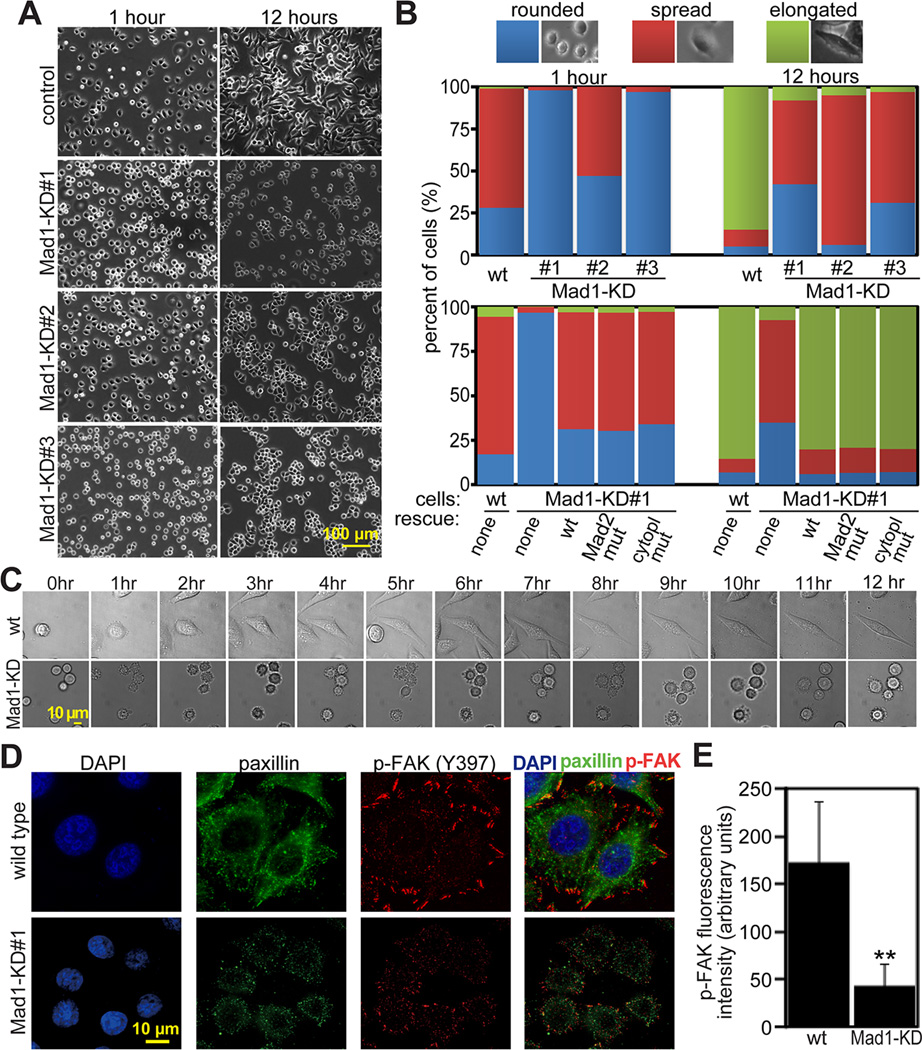
Figure 3. Mad1 depletion impairs cellular spreading and FAK-Tyr 397 phosphorylation.
(A) Mad1-KD cell lines #1–3 are substantially less well spread than wild type HeLa cells 1 and 12 hours after plating on fibronectin.
(B) Quantification of cellular phenotype 1 and 12 hours after plating on fibronectin coated dishes. n>300 cells from each of 3 independent experiments. Top, wild type and Mad1-KD HeLa cell lines 1–3. Bottom, wild type and Mad1-KD#1 cells transfected with the indicated shRNA-resistant rescue construct. “Mad2 mut” indicates Mad1 containing the mutations K541A/L543A, which is unable to bind Mad2 [26]. “Cytopl mut” indicates a cytoplasmic Mad1 mutant (aa 180–718) lacking the nuclear import signal [12]. All rescue contructs were GFP-tagged.
(C) Still images from timelapse analysis of wild type and Mad1-KD#1 HeLa cells showing that wild type cells begin spreading by 3 hours while Mad1-KD cells remain rounded 12 hours after plating on fibronectin. See also Supplemental Movie 2.
(D) Reduction of Mad1 results in reduced phosphorylation of FAK on Tyr 397 4 hours after plating on fibronectin coated coverslips.
(E) Quantification of fluorescence intensity +/− SD of phospho-FAK (Tyr 397) in wild type and Mad1-KD#1 cells, as pictured in D. n=40 cells from each of 3 independent experiments. **=p<0.001.