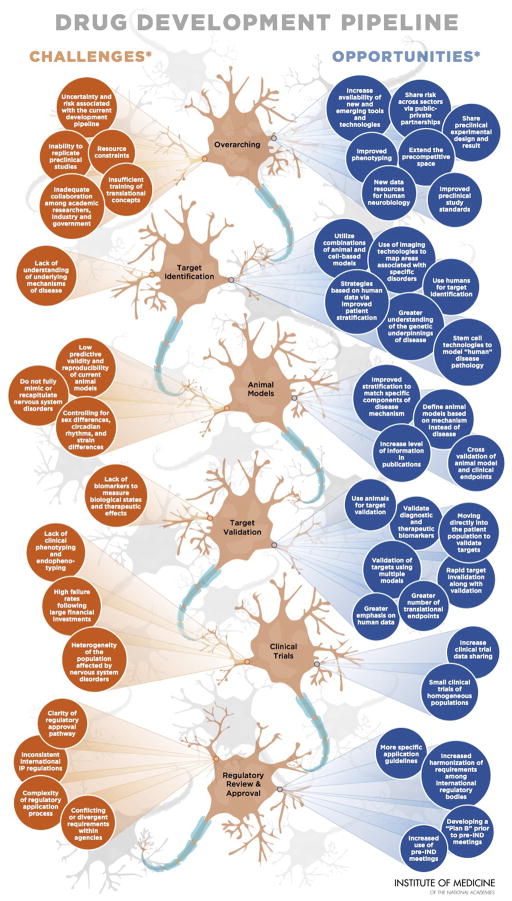
Figure 1. Improving and Accelerating Therapeutic Development for Nervous System Disorders.
Although there is a high burden associated with nervous system disorders, development of new therapeutics remains stagnant. Over the last decade, fewer new drugs for nervous system disorders have garnered approval in comparision to other therapeutic areas. Current data suggest that drug development, from the start of a discovery program to regulatory approval, can take an average of 12 to 15 years. This familiar statistic prompts an equally familiar question: Can the therapeutic development timeline be improved and accelerated by addressing challenges and developing opportunities? The challenges and opportunities presented in this graphic are not related and are presented in no certain order. The opportunities listed should not be interpreted as solutions to the challenges identified.
This graphic illustrates common challenges and opportunities suggested by individual participants in workshops hosted by the IOM Forum on Neuroscience and Nervous System Disorders (Accelerating Therapeutic Development for Nervous System Disorders Towards First-in-Human Trials [April 8–9, 2013] and improving Translation of Animal Models for Nervous System Disorders (March 28–29, 2012]). Statements, recommendations, and opinions expressed are those of the individual participants and are not necessarily endorsed or verified by the IOM, and should not be construed as reflecting any group consensus.
IND, investigational new drug; IP, intellectual property. Adapted and reprinted with permission from the Institute of Medicine.