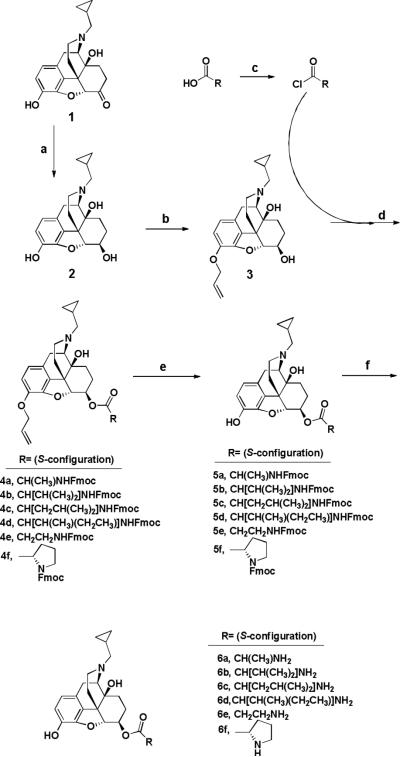
Scheme 1.
Synthesis of amino acid ester prodrugs of 6-β-naltrexol: (a) formamidinesulfinic acid (4.0 eq), aqueous NaOH (0.53 M), 80°C, 1.5 h;24 (b) allyl bromide (1.1 eq), K2CO3 (4 eq), acetone, reflux, 5.5 h; (c) Fmoc-protected amino acids (2.0 eq), SOCl2 (1.99 eq), sonicate 30 min, DMAP (2.0 eq), argon stream; (d) DMAP (2.0 eq), DCM, 0°C - rt, 4 h, argon atmosphere; (e) PMHS (3.0 eq), 2M ZnCl2 in Et2O (52 drops via syringe/100 mg 2), (PPh3)4Pd (5 mol%), THF, rt, 24 h, argon atmosphere; (f) Octanethiol (10 eq), DBU (25 mol%), THF, rt, 24 h, argon atmosphere.