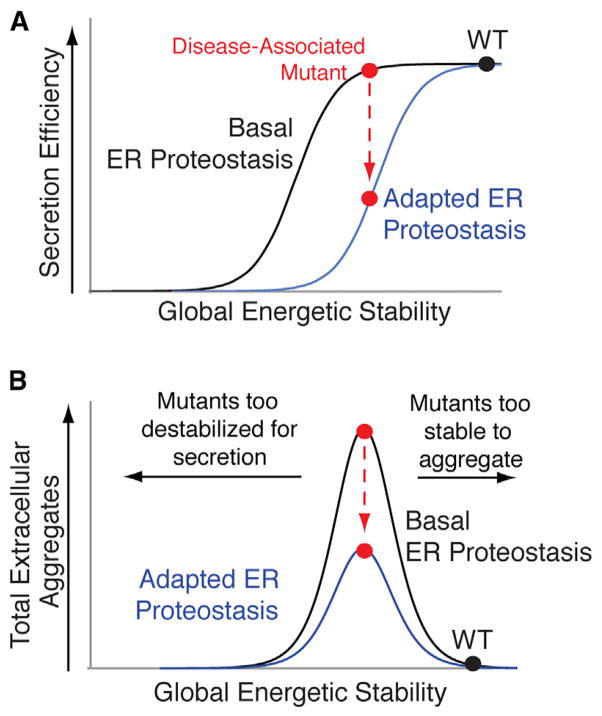
Figure 7. Increasing ER Quality Control Stringency Is a Viable Therapeutic Approach to Reduce Extracellular Concentrations of Proteotoxic Aggregates.
(A) Illustration showing that adapting the ER proteostasis environment to enhance ER quality control stringency can reduce secretion of destabilized amyloidogenic protein variants (red) without significantly impacting secretion of stable, WT protein (black).
(B) Illustration showing that increasing ER quality control stringency through the adaption of the ER proteostasis environment provides a therapeutic approach to preferentially reduce the extracellular aggregation of destabilized, aggregation-prone protein variants most commonly associated with disease pathogenesis (red). Highly destabilized protein variants are recognized by ER quality control pathways, increasing their degradation in the ER, decreasing their secretion to the extracellular space, and reducing extracellular concentrations available for concentration-dependent aggregation (left arrow). Alternatively, stable protein variants prevent the initial misfolding steps required for protein aggregation (right arrow).