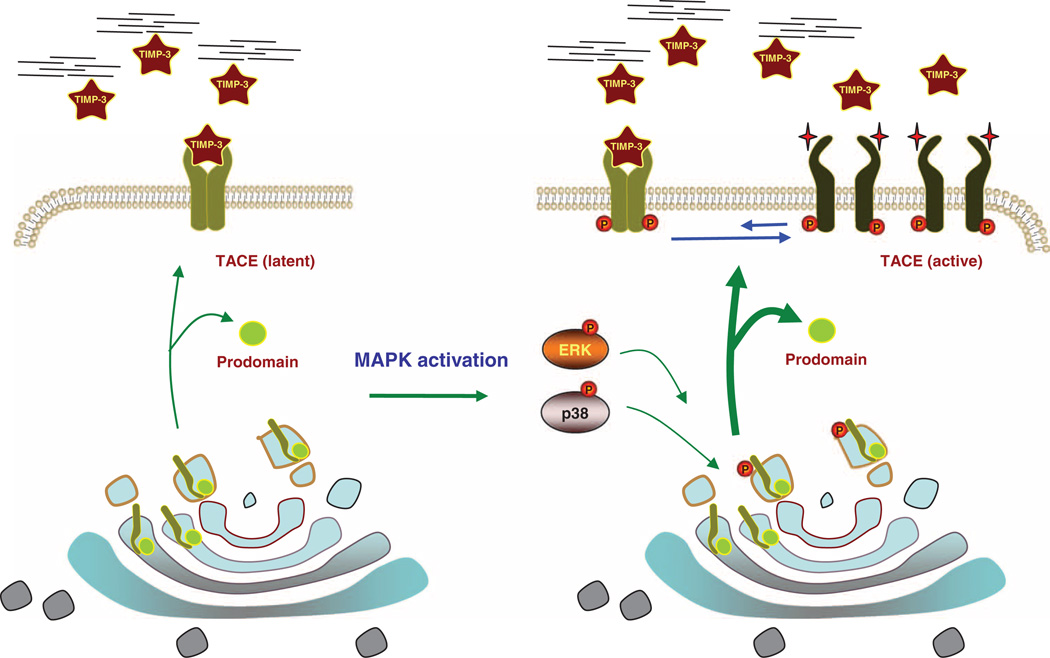
Fig. 7.
Model of TACE activation by MAPK signaling. Without MAPK signaling, cell surface TACE is primarily present as dimer and readily associates with TIMP3, possibly binding to the TACE dimer in a 2:2 ratio. TACE is therefore only partially active and its activity depends on the availability of TIMP3 (left). Upon p38 or ERK MAPK pathway activation, the cytoplasmic domain of TACE is phosphorylated, resulting in rapidly increased cell surface presentation (wider arrows), higher abundance of TACE monomers, and lower abundance of dimers. The lack of dimerization results in lower association of TACE with TIMP3 and the activation of TACE (right). The activity of TACE thus results from the dynamic balance between TACE dimers and monomers at the cell surface and the resulting differences in TIMP3 binding efficiency.