Figure 4.
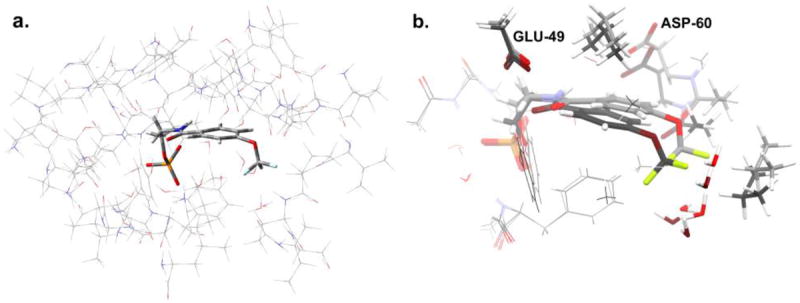
a. Model of the active site in the α-subunit of tryptophan synthase, showing (thin wireframe) the side chains fixed at their crystallographically-determined coordinates and (thick wireframe) the F6-substrate. The structure shown corresponds to the (F6)(Na+)E(Q)2AP form; an analogous model was built for (F6)(Na+)E(Ain). b: Superposition of the geometry-optimized F6 substrates for (F6)(Na+)E(Ain) (light gray carbon atoms) and (F6)(Na+)E(Q)2AP (dark gray carbon atoms) forms, also showing residue fragments within 3 Å of the CF3 group. These substructures were used for calculating NMR chemical shifts. The standard CPK scheme is used to designate the atom colors (H, white; C, gray; N, blue; F: green; O, red; P, orange; S, yellow).
