Chart 1.
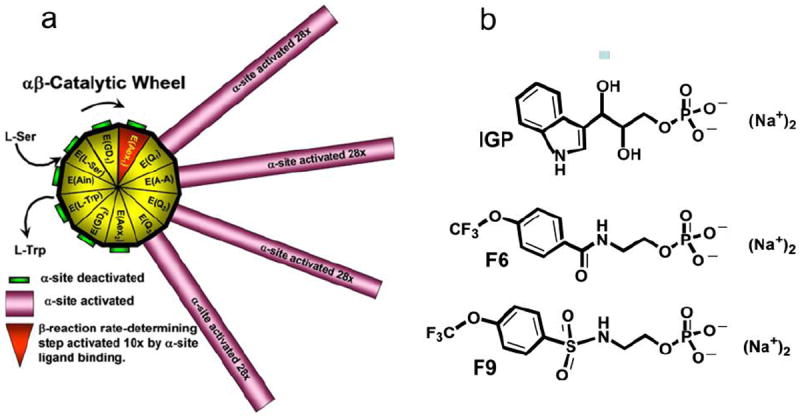
a Representation of the chemical and conformational events which synchronize the α-and β- catalytic activities of αβ-dimeric units of tryptophan synthase and prevent the escape of indole. The 11 chemical states of the β-reaction are depicted as triangles around the hub of the catalytic wheel. The spokes connected to the triangles of the hub represent the activity states of the α-site as the β-site cycles through the 11 chemical states. The α-subunit is switched between inactive (green) and active (magenta) conformations in response to the interconversion among covalent intermediates along the β-site catalytic path. This switching activates or deactivates the α-site by ~28-fold. Activation of the α-site occurs when the L-Ser external aldimine, E(Aex1) is converted to the α-aminoacrylate Schiff base, E(A-A), while deactivation occurs when the quinonoid intermediate, E(Q3), is converted to the L-Trp external aldimine, E(Aex2). During Stage I of the β-reaction the rate-limiting conversion of E(Aex1) (red triangle) to E(A-A) is activated at least 10-fold by IGP binding to the α-site. (Figure taken from Dunn et al.11). This switching between low (open) and high (closed) activity states synchronizes the α- and β-catalytic activities, preventing the escape of indole as it is transferred between the α- and β-sites. b: Structures of N-(4’-trifluoromethoxybenzoyl)-2-aminoethyl phosphate (F6), and N-(4’-trifluoromethoxybenzenesulfonyl)-2-aminoethyl phosphate (F9).
